NCERT Solutions for Class 11 Chemistry Chapter 7 Redox Reactions
NCERT Solutions for Class 11 Chemistry Chapter 7
The NCERT Solutions for Redox reaction Class 11 Chemistry Chapter 7 are collated by the top subject-matter experts at SimplyAcad to assist students in their study. The syllabus is in accordance with the recent syllabus, students will gain deep in-sights of all the important concepts discussed in the chapter. The solutions are in simple and easy to understand language, so that clarity is maintained while learning such complex topics.
NCERT Solutions for Class 11 Chemistry Chapter 7 Redox Reaction is a crucial segment in the syllabus prescribed by CBSE. These solutions will be useful for students preparing for the various upcoming entrance engineering and medical entrances.
Access the NCERT Solutions for Class 11 Chemistry Chapter 7 Redox Reaction
Question 1. Assign oxidation number to the underlined elements in each of the following species:

Answer:

Question 2. What are the oxidation numbers of the underlined elements in each of the following and how do you rationalize your results? \begin{enumerate} \item \(\text{KI}_3\) \item \(\text{H}_2\text{S}_4\text{O}_6\) \item \(\text{Fe}_3\text{O}_4\) \item \(\text{C}_2\text{H}_3\text{COOH}\) \item \(\text{C}_2\text{H}_4\text{O}_2\) \end{enumerate} \section*
{Solution} \begin{enumerate} \item \textbf{KI}_3 In \(\text{KI}_3\), the oxidation number (O.N.) of \( \text{K} \) is \( +1 \). The average oxidation number of \( \text{I} \) is: \[ \text{Average O.N. of I} = \frac{-1 \times 3}{3} = -1 \] However, this average O.N. does not accurately reflect the oxidation states in the molecule. In \(\text{KI}_3\), iodine forms a coordinate covalent bond with another iodine molecule, leading to different oxidation states for iodine atoms: \[ \text{KI}_3: \quad +1 \text{ (for K)} + [0 \text{ (for } \text{I}_2) \text{ and } -1 \text{ (for } \text{I}_{\text{coordinate}})] \] Hence, in \(\text{KI}_3\), the O.N. of the iodine atoms is \( 0 \) for those in \(\text{I}_2\) and \(-1\) for the iodine involved in the coordinate bond. \item \textbf{H}_2\text{S}_4\text{O}_6 The general formula for the oxidation state is: \[ 2(\text{H}) + 4(\text{S}) + 6(\text{O}) = 0 \] Substituting known values, where \(\text{O} = -2\): \[ 2 \times (+1) + 4 \times x + 6 \times (-2) = 0 \] \[ 2 + 4x – 12 = 0 \] \[ 4x = 10 \implies x = +2.5 \] Since oxidation numbers cannot be fractional, sulfur must be present in different oxidation states. Typically, in \(\text{H}_2\text{S}_4\text{O}_6\), sulfur exhibits oxidation states of \(+5\) and \(0\). \item \textbf{Fe}_3\text{O}_4 For \(\text{Fe}_3\text{O}_4\), using \(\text{O} = -2\): \[ 3(\text{Fe}) + 4(-2) = 0 \] \[ 3x – 8 = 0 \implies x = +\frac{8}{3} = +2.67 \] Since oxidation numbers must be integer values, in \(\text{Fe}_3\text{O}_4\), one iron atom exhibits \(+2\) and the other two exhibit \(+3\). Thus, \(\text{Fe}_3\text{O}_4\) can be considered as a mixture of \(\text{FeO}\) and \(\text{Fe}_2\text{O}_3\). \item \textbf{C}_2\text{H}_3\text{COOH} The oxidation state of carbon in \(\text{C}_2\text{H}_3\text{COOH}\) is calculated by: \[ 2(x) + 3(+1) + 2(-2) = 0 \] \[ 2x + 3 – 4 = 0 \implies 2x – 1 = 0 \implies x = +2 \] Thus, the oxidation states of carbon in \(\text{C}_2\text{H}_3\text{COOH}\) are \(+2\) and \(0\). \item \textbf{C}_2\text{H}_4\text{O}_2 For \(\text{C}_2\text{H}_4\text{O}_2\): \[ 2x + 4(+1) + 2(-2) = 0 \] \[ 2x + 4 – 4 = 0 \implies 2x = 0 \implies x = 0 \] Thus, in \(\text{C}_2\text{H}_4\text{O}_2\), carbon exhibits an average oxidation state of \(0\), but in different environments, it can show states of \(+2\) and \(-2\).
Question 3. Justify that the following reactions are redox reactions:
(a) CuO(s) + H2(g) —–> Cu(s) + H20(g)
(b) Fe2O3(s) +3CO(g) —-> 2Fe(s) + 3CO2(g)
(c) 4BCl3(g) +3LiAlH4(s) ——> 2B2H6(g) + 3LiCl(s) + 3AlCl3(s)
(d) 2K(s) +F2(g)——> 2K+F–(s)
Answer:
![]()
Here, O is removed from CuO, therefore, it is reduced to Cu while O is added to H2 to form H20, therefore, it is oxidised. Further, O.N. of Cu decreases from + 2 in CuO to 0 in Cu but that of H increases from 0 in H2 to +1 in H20. Therefore, CuO is reduced to Cu but H2 is oxidised to H20. Thus, this is a redox reaction.

Here O.N. of Fe decreases from +3 if Fe2O3 to 0 in Fe while that of C increases from +2 in CO to +4 in CO2. Further, oxygen is removed from Fe2O3 and added to CO, therefore, Fe2O3 is reduced while CO is oxidised. Thus, this is a redox reaction.
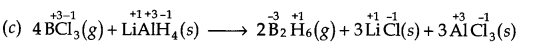
Here, O.N. of B decreases from +3 in BrCl3to -3 in B2H6 while that of H increases from -1 in LiAlH4to +1 in B2H6. Therefore, BCl3 is reduced while LiAlH4 is oxidised. Further, H is added to BCl3 but is removed from LiAlH4, therefore, BC13 is reduced while LiAlH4 is oxidised. Thus, it is a redox reaction.
Here, each K atom as lost one electron to form K+ while F2 has gained two electrons to form two F– ions. Therefore, K is oxidised while F2 is reduced. Thus, it is a redox reaction.
By chemical bonding, C2 is attached to three H-atoms (less electronegative than carbon) and one CH2OH group (more electronegative than carbon), therefore,
O.N. of C2 = 3 (+1) + x + 1 (-1) = 0 or x = -2 C2 is, however, attached to one OH (O.N. = -1) and one CH3 (O.N. = +1) group, therefore, O.N. of C4 = + 1 + 2 (+1) + x + 1 (-1) = 0 or x = -2
Question 4. Fluorine reacts with ice and results in the change:
H20(S) + F2 (g) ——-> HF(g) + HOF(g)
Justify that this reaction is a redox reaction.
Answer: Writing the O.N. of each atom above its symbol, we have,
![]()
Here, the O.N. of F decreases from 0 in F2 to -1 in HF and increases from 0 in F2 to +1 in HOF. Therefore, F2 is both reduced as well as oxidised. Thus, it is a redox reaction and more specifically, it is a disproportionation reaction.
Question 5. Calculate the oxidation number of sulfur, chromium, and nitrogen in \(\text{H}_2\text{SO}_5\), \(\text{Cr}_2\text{O}_7^{2-}\), and \(\text{NO}_3^-\). Suggest the structure of these compounds and identify any fallacies. \section*
{Solution} \begin{enumerate} \item \textbf{\(\text{H}_2\text{SO}_5\)} By the conventional method, let \(x\) be the oxidation number of sulfur (S). We have: \[ 2(+1) + x + 5(-2) = 0 \] \[ 2 + x – 10 = 0 \] \[ x = +8 \] An oxidation state of +8 for sulfur is not possible as sulfur cannot have an oxidation number greater than +6. To overcome this fallacy, we need to use the structure of the compound. The correct structure is: \[ \text{H} – \text{O} – \text{S}(O_2) – \text{O} – \text{O} – \text{H} \] Using this structure: \[ -1 + x + 2(-2) + 2(-1) + 1 = 0 \] \[ -1 + x – 4 – 2 + 1 = 0 \] \[ x = +6 \] Hence, the oxidation number of sulfur in \(\text{H}_2\text{SO}_5\) is +6. \item \textbf{\(\text{Cr}_2\text{O}_7^{2-}\)} Let \(x\) be the oxidation number of chromium (Cr). For the dichromate ion, we have: \[ 2x + 7(-2) = -2 \] \[ 2x – 14 = -2 \] \[ 2x = +12 \] \[ x = +6 \] Hence, the oxidation number of chromium in the dichromate ion is +6. There is no fallacy in this calculation. \item \textbf{\(\text{NO}_3^-\)} Let \(x\) be the oxidation number of nitrogen (N). For the nitrate ion, we have: \[ x + 3(-2) = -1 \] \[ x – 6 = -1 \] \[ x = +5 \] Using the structure: \[ \text{O} – \text{N} (+) – (\text{O}) – \text{O}^- \] The oxidation number calculation confirms: \[ x + 1(-1) + 2(-2) = -1 \] \[ x – 1 – 4 = -1 \] \[ x = +5 \] Thus, there is no fallacy, and the oxidation number of nitrogen in \(\text{NO}_3^-\) is +5. \end{enumerate}
Question 6.Write formulas for the following compounds:
(a) Mercury (II) chloride, (b) Nickel (II) sulphate, (c) Tin (IV) oxide, (d) Thallium
(I) sulphate, (e) Iron (III) sulphate, (f) Chromium (III) oxide.
Answer: (a) Hg(II)Cl2, (b) Ni(II)SO4, (c)Sn(IV)O2 (d) T12(I)SO4, (e) Fe2(III)(S04)3, (f) Cr2(III)O3.
Question 7. Suggest a list of substances where carbon can exhibit oxidation states from -4 to +4 and nitrogen from -3 to +5.
Answer:

Question 8. While sulphur dioxide and hydrogen peroxide can act as an oxidising as well as reducing agents in their reactions, ozone and nitric acid act only as oxidants. Why?
Answer: (i) In S02 , O.N. of S is +4. In principle, S can have a minimum O.N. of -2 and maximum of +6. Therefore, S in S02 can either decrease or increase its O.N. and hence can act both as an oxidising as well as a reducing agent.
(ii) In H2O2, the O.N. of O is -1. In principle, O can have a minimum O.N. of -2 and maximum of zero (+1 is possible in O2F2and +2 in OF2). Therefore, O in H2O2 can either decrease its O.N. from -1 to -2 or can increase its O.N. from -1 to zero. Therefore, H2O2 acts both as an oxidising as well as a reducing agent.
(iii) In O3, the O.N. of O is zero. It can only decrease its O.N. from zero to -1 or -2, but cannot increase to +2. Therefore, O3 acts only as an oxidant.
(iv) In HNO3, O.N. of N is +5 which is maximum. Therefore, it can only decrease its O.N. and hence it acts as an oxidant only.
Question 9. Why is it more appropriate to write the reaction: \[ \text{O}_3(g) + \text{H}_2\text{O}_2(l) \to \text{H}_2\text{O}(l) + 2 \text{O}_2(g) \] as: \[ \text{O}_3(g) + \text{H}_2\text{O}_2(l) \to \text{H}_2\text{O}(l) + \text{O}_2(g) + \text{O}_2(g)? \] Also, suggest a technique to investigate the path of the above redox reaction. \section*
{Solution} Oxygen is produced from ozone and hydrogen peroxide. To better understand the reaction mechanism, it is useful to consider the following reactions: \begin{align*} \text{O}_3(g) &\to \text{O}_2(g) + \text{O}(g) \quad \text{(1)} \\ \text{H}_2\text{O}_2(l) + \text{O}(g) &\to \text{H}_2\text{O}(l) + \text{O}_2(g) \quad \text{(2)} \end{align*} Adding reactions (1) and (2) gives the overall reaction: \[ \text{O}_3(g) + \text{H}_2\text{O}_2(l) \to \text{H}_2\text{O}(l) + \text{O}_2(g) + \text{O}_2(g) \] Thus, it is more appropriate to write the reaction as: \[ \text{O}_3(g) + \text{H}_2\text{O}_2(l) \to \text{H}_2\text{O}(l) + \text{O}_2(g) + \text{O}_2(g) \] This representation accurately reflects the individual steps and intermediates in the reaction, which helps in understanding the mechanism. To investigate the path of the above redox reaction, one can use isotopic labeling. For example, using \(\text{H}_2\text{O}^{18}\text{O}_2\) (with \(^{18}\text{O}\) as the isotope) in the reaction allows for tracking the fate of oxygen atoms during the reaction. By analyzing the products, one can determine whether the oxygen atoms from hydrogen peroxide are incorporated into water or oxygen gas, thus elucidating the reaction mechanism.
Question 10. The compound AgF2 is unstable. However, if formed, the compound acts as a very strong oxidising agent. Why?
Answer: In AgF2 oxidation state of Ag is +2 which is very very unstable. Therefore, it quickly accepts an electron to form the more stable +1 oxidation state.
Ag2+ + e– ————–> Ag+
Therefore, AgF2, if formed, will act as a strong oxidising agent.
Question 11. Whenever a reaction between an oxidising agent and a reducing agent is carried out, a compound of lower oxidation state is formed if the reducing agent is in excess and a compound of higher oxidation state is formed if oxidising agent is in excess. Justify this statement giving three illustrations.
Answer: (i) C is a reducing agent while O2 is an oxidising agent. If excess of carbon is burnt in a limited supply of O2, CO is formed in which the oxidation state of C is +2. If, however, excess of O2 is used, the initially formed CO gets oxidised to CO2 in which oxidation state of C is + 4.

(ii) P4 is a reducing agent while Cl2 is an oxidising agent. When excess of P4 is used, PCl3 is formed in which the oxidation state of P is + 3. If, however, excess of Cl2 is used, the initially formed PCl3 reacts further to form PCl5 in which the oxidation state of P is +5
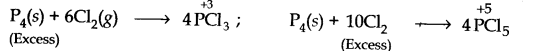
(iii) Na is a reducing agent while 02 is an oxidising agent. When excess of Na is used, sodium oxide is formed in which the oxidation state of O is -2. If, however, excess of 02 is used, Na2O2 is formed in which the oxidation state of O is -1 which is higher than -2.
![]()
Question 12. How do you account for the following observations?
(a) Though alkaline potassium permanganate and acidic potassium permanganate both are used as oxidants, yet in the manufacture of benzoic acid from toluene we use alcoholic potassium permanganate as an oxidant. Why? Write a balanced redox equation for the reaction.
(b) When concentrated sulphuric acid is added to an inorganic mixture containing chloride, we get colourless pungent smelling gas HCl, but if the mixture contains bromide then we get red vapour of bromine. Why?
Answer: (a) Toluene can be oxidised to benzoic acid in acidic, basic and neutral media according to the following redox equations:

In the laboratory, benzoic acid is usually prepared by alkaline KMnO4 oxidation of toluene. However, in industry alcoholic KMnO4 is preferred over acidic or alkaline KMnO4 because of the following reasons:
(i) The cost of adding an acid or the base is avoided because in the neutral medium, the base (OH- ions) are produced in the reaction itself.
(ii) Since reactions occur faster in homogeneous medium than in heterogeneous medium, therefore, alcohol helps in mixing the two reactants, i.e., KMnO4 (due to its polar nature) and toluene (because of its being an organic compound).
(b) When cone. H2S04 is added to an inorganic mixture containing chloride, a pungent smelling gas HCl is produced because a stronger acid displaces a weaker acid from its salt.

Since HCl is a very weak reducing agent, it can not reduce H2S04 to S02 and hence HCl is not oxidised to Cl2.
However, when the mixture contains bromide ion, the initially produced HBr being a strong reducing agent than HCl reduces H2S04to S02 and is itself oxidised to produce red vapour of Br2.

Question 13. Identify the substance oxidised, reduced, oxidising agent and reducing agent for each of the following reactions.

Answer:

Question 14. Consider the reactions:

Why does the same reductant, thiosulphate react difforerently with iodine and bromine?
Answer: The average O.N. of S in S2O32- is +2 while in S4O62- it is + 2.5. The O.N. of S in SO42- is +6. Since Br2 is a stronger oxidising agent that I2, it oxidises S of S2O32- to a higher oxidation state of +6 and hence forms SO42- ion. I2, however, being weaker oxidising agent oxidises S of S2O32- ion to a lower oxidation of +2.5 in S4O62- ion. It is because of this reason that thiosulphate reacts differently with Br2 and I2.
Question 15. Justify-giving reactions that among halogens, fluorine is the best oxidant and among hydrohalic compounds, hydroiodic add is the best reductant.
Answer: Halogens have a strong tendency to accept electrons. Therefore, they are strong oxidising agents. Their relative oxidising power is, however, measured in terms of their electrode potentials. Since the electrode potentials of halogens decrease in the order: F2 (+2.87V) > Cl2 (+1.36V) > Br2 (+1.09V) > I2 (+0.54V), therefore, their oxidising power decreases in the same order.
This is evident from the observation that F2 oxidises Cl– to Cl2, Br–to Br2, I – to I2 ; Cl2 oxidises Br–to Br2 and F to I2 but not F– to F2. Br2, however, oxidises F to I2 but not F– to F2 , and Cl– to Cl2.
F2(g) + 2Cr(aq) ———–> 2F–(aq) + Cl2(g); F2(g) + 2Br–(aq) ———-> 2F–(aq) + Br2 (Z)
F2(g) + 2I–(aq) ———-> 2F–(aq) + I2(s); Cl2 (g) + 2Br–(aq) ————> 2Cl–(aq) + Br2 (Z)
Cl2(g) + 2I–(aq) ———–> 2Cl– (aq) + I2(s) and Br2 (Z) + 2F ———> 2Br– (aq) + I2(s)
Thus, F2 is the best oxidant.
Conversely, halide ions have a tendency to lose electrons and hence can act as reducing agents. Since the electrode potentials of halide ions decreases in the order: I–(-0.54 V) > Br– (-1.09 V) > Cl–(-1.36 V) > I2 (-2.87 V), therefore, the reducing power of the halide ions or their corresponding hydrohalic acids decreases in the same order: HI > HBr > HCl > HF. Thus, hydroiodic acid is the best reductant. This is supported by the following reactions. For example, HI and HBr reduce H2S04 to S02 while HCl and HF do not.
2HBr + H2S04 —–> Br2+ S02 + 2H2O; 2HI + H2S04 ——> I2 + S02 + 2H2O
Further F reduces Cu2+ to Cu+ but Br does not.
2Cu2+(aq) + 4I–(aq) >Cu2I2(s) + I2(aq); Cu2+(aq) + 2Br–> No reaction.
Thus, HI is a stronger reductant than HBr.
Further among HCl and HF, HCl is a stronger reducing agent than HF because HCl reduces MnO2 to Mn2+ but HF does not.
MnO2 (s) + 4HCl(aq) ——-> MnCl2(aq) + Cl2(aq) + 2H2O
MnO2 (s) + 4HF(l) ———–> No reaction.
Thus, the reducing character of hydrohalic acids decreases in the order: HI > HBr > HCl > HF.
Question 16. Why does the following reaction occur?
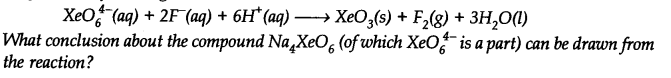
Answer:
Question 17. Consider the reactions:
(a) H3P02(aq) + 4AgNO3(aq) + 2H2O(l) ————->H3PO4(aq) + 4Ag(s) + 4HNO3(aq)
(b) H3P02(aq) + 2CuS04(aq) + 2H2O(l) ————->H3P04(aq) + 2Cu(s) + H2S04(aq)
(c) C6H5CHO(l) + 2[Ag(NH3)2]+(aq) + 30H–(aq)———–> C6H5COO–(aq) + 2Ag(s) + 4NH3(aq) + 2H20(l)
(d) C6H5CHO(l) + 2Cu2+(aq) + 5OH–(aq) ———–> No change observed
What inference do you draw about the behaviour of Ag+ and Cu2+ from these reactions?
Answer: Reactions (a) and (b) indicate that H3P02 (hypophosphorous acid) is a reducing agent and thus reduces both AgNO3 and CuS04 to Ag and Cu respectively. Conversely, both AgNO3 and CuS04 act as oxidising agent and thus oxidise H3P02to H3P04 (orthophosphoric acid) Reaction (c) suggests that [Ag(NH3)2]+ oxidises C6H5CHO (benzaldehyde) to C6H5COO– (benzoate ion) but reaction (d) indicates that Cu2+ ions cannot oxidise C6H5CHO to C6H5COO–. Therefore, from the above reactions, we conclude that Ag+ ion is a strong deoxidising agent than Cu2+ ion.
Question 18. Balance the following redox reactions by ion-electron method.
(a) MnO4–(aq) +I–(aq) ———>Mn02(s) + I2 (s) (in basic medium)
(b) MnO4–(aq) + S02(g) ——-> Mn2+(aq) +H2S04–(in acidic solution)
(c) H2O2(aq) + Fe2+(aq) ———-> Fe3+(aq) + H2O(l) (in acidic solution)
(d) Cr2O72- (aq) + S02 (g)——> Cr3+ (aq) + SO42-(aq) (in acidic solution)
Answer: (a) Do it yourself.
(b) The balanced half reaction equations are:
Oxidation half equation:
S02(g) + 2H2O(l) ——–> HS04– (aq) + 3H+(aq) +2e– …(i)
Reduction half equation:
MnO4–(aq) + 8H+(aq) + 5e– ——–> Mn2+(aq) + 4H2O(l) ………..(ii)
Multiply Eq. (i) by 3 and Eq. (ii) by 2 and add, we have,
2MnO4–(aq) + 5S02(g) + 2H20(l) + H+(aq) ————> 2Mn2+(aq) + 5HSO4–(aq)
(c) Oxidation half equation: Fe2+(aq) ———> Fe3+(aq) + e– …(i)
Reduction half equation: H2O2(aq) + 2H+(aq) + 2e– ———> 2H2O(l) …(ii)
Multiply Eq. (i) by 2 and add it to Eq. (ii), we have,
H2O2(aq) +2Fe2+(aq) +2H+(aq) ——-> 2Fe3+(aq) + 2H2O(l)
(d) Following the procedure detailed on page 8/23, the balanced half reaction equations are:
Oxidation half equation:
SO2(g) + 2H2O(l) ————> SO42-(aq) + 4H+(aq) + 2 e– …(i)
Reduction half equation:
Cr2O72–(aq) + 14H+(aq) + 6e– ————> 2Cr3+(aq) + 7H20(l) …(ii)
Multiply Eq. (i) by 3 and add it to Eq. (ii), we have,
Cr2O72–(aq) + 3SO2(q) + 2H+(aq) ————> 2Cr3+(aq) + 3SO42-(aq) + H20(l)
Question 19. Balance the following equation in basic medium by ion electron method and oxidation number method and identify the oxidising agent and the reducing agent.
(a) P4(s) + OH–(aq) ———> PH3(g) + H2PO2–(aq)
(b) N2H4(l) + ClO–(aq) ——–> NO(g) + CV(aq)
(c) Cl2O7(g) + H2O2(aq) ———-> ClO2–(aq) + O2(g) + H+
P4 acts both as an oxidising as well as a reducing agent.
Answer:






Question 20. Write Jour informations about the reaction:
(CN)2(g) + 2OH–(aq) —–> CN–(aq) + CNO–(aq) + H2O(l)
Answer: Let x be the O.N. of C.
O.N. of C in cyanogen, (CN)2 = 2 (x – 3) = 0 or x = +3 O.N. of C in cyanide ion, CN- = x – 3 = -1 or x = +2 O.N. of C in cyanate ion, CNO =x-3-2 = -lora: = +4 The four information about the reaction are:
(i) The reaction involves decomposition of cyanogen, (CN)2 in the alkaline medium to cyanide ion, CN and cyanate ion,CNO–.
(ii) The O.N. of C decreases from +3 in (CN)2 to +2 in CN–ion and increases from +3 in(CN)2 to +4 in CNO– ion. Thus, cyanogen is simultaneously reduced to cyanide ion and oxidised to cyanate ion.
(iii) It is an example of a redox reaction in general and a disproportionation reaction in particular.
(iv) Cyanogen is a pseudohalogen (behaves like halogens) while cyanide ion is a pseudohalide ion (behaves like halide ion).
Question 21. The Mn\(^{3+}\) ion is unstable in solution and undergoes disproportionation to give Mn\(^{2+}\), MnO\(_2\), and H\(^+\) ion. Write a balanced ionic equation for the reaction. \section*
{Solution} To balance the ionic equation for the disproportionation reaction of Mn\(^{3+}\), follow these steps: \begin{enumerate} \item \textbf{Write the unbalanced reaction:} \[ \text{Mn}^{3+} + \text{H}_2\text{O} \rightarrow \text{Mn}^{2+} + \text{MnO}_2 + \text{H}^+ \] \item \textbf{Balance manganese atoms:} Since there are two manganese atoms on the product side (one in MnO\(_2\) and one in Mn\(^{2+}\)), multiply the reactants by 2 to balance manganese atoms: \[ 2\text{Mn}^{3+} + \text{H}_2\text{O} \rightarrow \text{Mn}^{2+} + \text{MnO}_2 + \text{H}^+ \] \item \textbf{Balance oxygen and hydrogen atoms:} Adjust the number of water molecules and hydrogen ions to balance oxygen and hydrogen atoms: \[ 2\text{Mn}^{3+} + 2\text{H}_2\text{O} \rightarrow 2\text{Mn}^{2+} + \text{MnO}_2 + 4\text{H}^+ \] \item \textbf{Check for charge balance:} \begin{itemize} \item Total charge on the reactant side: \(2 \times (+3) = +6\) \item Total charge on the product side: \(2 \times (+2) = +4\) (Mn\(^{2+}\)) + \(4 \times (+1) = +4\) (H\(^+\)) \end{itemize} Both sides balance in terms of charge. \end{enumerate} \textbf{Balanced Ionic Equation:} \[ 2\text{Mn}^{3+} + 2\text{H}_2\text{O} \rightarrow 2\text{Mn}^{2+} + \text{MnO}_2 + 4\text{H}^+ \]
Question 22. Consider the elements: Cs, Ne, I, F
(a) Identify the element that exhibits -ve oxidation state.
(b) Identify the element that exhibits +ve oxidation state.
(c) Identify the element that exhibits both +ve and -ve oxidation states.
(d) Identify the element which neither exhibits -ve nor +ve oxidation state.
Answer: (a) F. Fluorine being the most electronegative element shows only a -ve oxidation state of -1.
(b) Cs. Alkali metals because of the presence of a single electron in the valence shell, exhibit an oxidation state of +1.
(c) I. Because of the presence of seven electrons in the valence shell, I shows an oxidation state of -1 (in compounds of I with more electropositive elements such as H, Na, K, Ca, etc.) or an oxidation state of +1 compounds of I with more electronegative elements, i.e., O, F, etc.) and because of the presence of d-orbitals it also exhibits +ve oxidation states of +3, +5 and +7.
(d) Ne. It is an inert gas (with high ionization enthalpy and high positive electron gain enthalpy) and hence it neither exhibits -ve nor +ve oxidation states.
Ques 23: Chlorine is used to purify drinking water. Excess chlorine is harmful. The excess chlorine is removed by treating with sulfur dioxide. Present a balanced equation for this redox change taking place in water. \section*
{Solution} The given redox reaction can be represented as: \[ \text{Cl}_2(s) + \text{SO}_2(aq) + \text{H}_2\text{O}(l) \rightarrow 2\text{Cl}^-(aq) + \text{SO}_4^{2-}(aq) \] \subsection*{Oxidation Half-Reaction} The oxidation half-reaction is: \[ \text{SO}_2(aq) \rightarrow \text{SO}_4^{2-}(aq) + 2e^- \] Balancing the oxygen atoms and hydrogen ions: \[ \text{SO}_2(aq) + 2\text{H}_2\text{O}(l) \rightarrow \text{SO}_4^{2-}(aq) + 4\text{H}^+(aq) + 2e^- \] \subsection*{Reduction Half-Reaction} The reduction half-reaction is: \[ \text{Cl}_2(s) + 2e^- \rightarrow 2\text{Cl}^-(aq) \] \subsection*{Balanced Chemical Equation} Adding the oxidation and reduction half-reactions together: \[ \text{Cl}_2(s) + \text{SO}_2(aq) + 2\text{H}_2\text{O}(l) \rightarrow 2\text{Cl}^-(aq) + \text{SO}_4^{2-}(aq) + 4\text{H}^+(aq) \]
Question 24. Refer to the periodic table given in your book and now answer the following questions: \begin{enumerate} \item[(a)] Select the possible non-metals that can show disproportionation reactions. \item[(b)] Select three metals that can show disproportionation reactions. \end{enumerate} \section*
{Solution} In disproportionation reactions, one of the reacting substances always contains an element that can exist in at least three oxidation states. \begin{enumerate} \item[(a)] Non-metals that can show disproportionation reactions include: \begin{itemize} \item Phosphorus (P) \item Chlorine (Cl) \item Sulfur (S) \end{itemize} These elements can exist in three or more oxidation states. \item[(b)] Metals that can show disproportionation reactions include: \begin{itemize} \item Manganese (Mn) \item Copper (Cu) \item Gallium (Ga) \end{itemize} These elements can exist in three or more oxidation states. \end{enumerate}
Question 25. In Ostwald’s process for the manufacture of nitric add, the first step involves the oxidation of ammonia gas by oxygen gas to give nitric oxide gas and steam. What is the maximum wight of nitric oxide that can be obtained starting only with 10.0 g of ammonia and 20.0 g of oxygen?
Answer: The balanced equation for the reaction is:
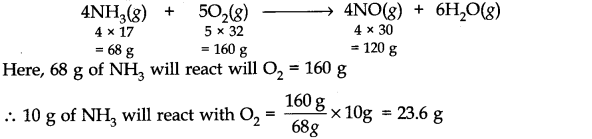
But the amount of O2 which is actually available is 20.0 g which is less than the amount which is needed. Therefore, 02 is the limiting reagent and hence calculations must be based upon the amount of 02 taken and not on the amount of NH3 taken. From the equation,
160 g of 02 produce NO = 120 g
.•. 20 g of 02 will produce NO =120/160 x 20 = 15 g
Question 26. Using the standard electrode potentials given in Table 3.1, predict if the reaction between the following is feasible: \begin{enumerate} \item[(i)] \(\text{Fe}^{3+}(\text{aq})\) and \(\text{I}^-(\text{aq})\) \item[(ii)] \(\text{Ag}^+(\text{aq})\) and \(\text{Cu}(\text{s})\) \item[(iii)] \(\text{Fe}^{3+}(\text{aq})\) and \(\text{Br}^-(\text{aq})\) \item[(iv)] \(\text{Ag}(\text{s})\) and \(\text{Fe}^{3+}(\text{aq})\) \item[(v)] \(\text{Br}_2(\text{aq})\) and \(\text{Fe}^{2+}(\text{aq})\) \end{enumerate} \section*
{Solution} When the cell potential (\(E^\circ_{\text{cell}}\)) is positive, the reaction is feasible. \begin{enumerate} \item[(i)] For the reaction between \(\text{Fe}^{3+}(\text{aq})\) and \(\text{I}^-(\text{aq})\): \[ E^\circ_{\text{cell}} = E^\circ_{\text{Fe}^{3+}/\text{Fe}} – E^\circ_{\text{I}_2/\text{I}^-} \] Given: \[ E^\circ_{\text{Fe}^{3+}/\text{Fe}} = 0.77\, \text{V} \] \[ E^\circ_{\text{I}_2/\text{I}^-} = 0.54\, \text{V} \] \[ E^\circ_{\text{cell}} = 0.77 – 0.54 = 0.23\, \text{V} \] The reaction is feasible. \item[(ii)] For the reaction between \(\text{Ag}^+(\text{aq})\) and \(\text{Cu}(\text{s})\): \[ E^\circ_{\text{cell}} = E^\circ_{\text{Ag}^+/\text{Ag}} – E^\circ_{\text{Cu}^{2+}/\text{Cu}} \] Given: \[ E^\circ_{\text{Ag}^+/\text{Ag}} = 0.80\, \text{V} \] \[ E^\circ_{\text{Cu}^{2+}/\text{Cu}} = 0.34\, \text{V} \] \[ E^\circ_{\text{cell}} = 0.80 – 0.34 = 0.46\, \text{V} \] The reaction is feasible. \item[(iii)] For the reaction between \(\text{Fe}^{3+}(\text{aq})\) and \(\text{Br}^-(\text{aq})\): \[ E^\circ_{\text{cell}} = E^\circ_{\text{Fe}^{3+}/\text{Fe}} – E^\circ_{\text{Br}_2/\text{Br}^-} \] Given: \[ E^\circ_{\text{Fe}^{3+}/\text{Fe}} = 0.77\, \text{V} \] \[ E^\circ_{\text{Br}_2/\text{Br}^-} = 1.09\, \text{V} \] \[ E^\circ_{\text{cell}} = 0.77 – 1.09 = -0.32\, \text{V} \] The reaction is not feasible. \item[(iv)] For the reaction between \(\text{Ag}(\text{s})\) and \(\text{Fe}^{3+}(\text{aq})\): \[ E^\circ_{\text{cell}} = E^\circ_{\text{Fe}^{3+}/\text{Fe}} – E^\circ_{\text{Ag}^+/\text{Ag}} \] Given: \[ E^\circ_{\text{Fe}^{3+}/\text{Fe}} = 0.77\, \text{V} \] \[ E^\circ_{\text{Ag}^+/\text{Ag}} = 0.80\, \text{V} \] \[ E^\circ_{\text{cell}} = 0.77 – 0.80 = -0.03\, \text{V} \] The reaction is not feasible. \item[(v)] For the reaction between \(\text{Br}_2(\text{aq})\) and \(\text{Fe}^{2+}(\text{aq})\): \[ E^\circ_{\text{cell}} = E^\circ_{\text{Br}_2/\text{Br}^-} – E^\circ_{\text{Fe}^{2+}/\text{Fe}} \] Given: \[ E^\circ_{\text{Br}_2/\text{Br}^-} = 1.09\, \text{V} \] \[ E^\circ_{\text{Fe}^{2+}/\text{Fe}} = 0.77\, \text{V} \] \[ E^\circ_{\text{cell}} = 1.09 – 0.77 = 0.32\, \text{V} \] The reaction is feasible.
Question 27. Predict the products of electrolysis in each of the following:
(i) An aqueous solution of AgNO3 with silver electrodes.
(ii) An aqueous solution of silver nitrate with platinum electrodes.
(iii) A dilute solution of H2S04with platinum electrodes.
(iv) An aqueous solution of CuCl2 with platinum electrodes.
Answer: (i) In aqueous solution, AgNO3 ionises to give Ag+(aq) and NO3– (aq) ions.
AgN03(aq) ——–> Ag+(aq) + NO3– (aq)
Thus, when electricity is passed, Ag+(aq) ions move towards the cathode while NO3– ions move towards the anode. In other words, at the cathode, either Ag+(aq) ions or H2O molecules may be reduced. Which of these will actually get discharged would depend upon their electrode potentials which are given below:
Ag+(aq) +e–———-> Ag(s); E° = +0.80 V …(i)
2H2O(Z) + 2e– ————> H2(g) + 2OH–(aq); E° = -0.83 V …(ii)
Since the electrode potential (i.e., reduction potential of Ag+(aq) ions is higher than that of H2O molecules, therefore, at the cathode, it is the Ag+(aq) ions (rather than H2O molecules) which are reduced.
Similarly, at the anode, either Ag metal of the anode or H2O molecules may be oxidised. Their electrode potentials are:
Ag(s) ———–> Ag+(aq) + e–; E° = -0.80 V …(iii)
2H2O(l) ————–> 02(g) +4H+(aq)+4e– ; E° = -1.23 V …(iv)
Since the oxidation potential of Ag is much higher than that of H2O, therefore,
at the anode, it is the Ag of the silver anode which gets oxidised and not the H2O molecules. It may, however, be mentioned here that the oxidation potential of N03–ions is even lower than that of H2O since more bonds are to broken during reduction of N03 ions than those in H2O.
Thus, when an aqueous solution 0f AgN03 is electrolysed, Ag from Ag anode dissolves while Ag+(aq) ions present in the solution get reduced and get deposited on the cathode.
(ii) If, however, electrolysis of AgN03 solution is carried out using platinum electrodes, instead of silver electrodes, oxidation of water occurs at the anode since Pt being a noble metal does not undergo oxidation easily. As a result, O2 is liberated at the anode according to equation (iv).
Thus, when an aqueous solution of AgNO3 is electrolysed using platinum electrodes, Ag+ ions from the solution get deposited on the cathode while 02 is liberated at the anode.
(iii) In aqueous solution, H2S04ionises to give H+(aq) and SO42-(aq) ions.
H2S04(aq) ——> 2H+(aq) +S04–(aq)
Thus, when electricity is passed, H+ (aq) ions move towards cathode while SO42-(aq) ions move towards anode. In other wode either H+(aq) ions or H2O molecules are reduced. Their electrode potentials are:2H+(aq)2e– ——-> H2(g); E° = 0.0 V
H2O(aq) + 2e– ——-> H2(g) + 2OH–((aq); E° = -0.83 V
Since the electron potential (i.e., reduction potential) of H+(aq) ions is higher than that of H2O, therefore, at the cathode, it is H+(aq) ions (rather than H2O molecules) which are reduced to evolve H2 gas.
Similarly at the anode, either SO42-(aq) ions or H2O molecules are oxidised. Since the oxidation potential of SO4 is expected to be much lower (since it involved cleavage of many bonds as compared to those in H20) than that of HjO molecules, therefore, at the anode, it is H2O molecules (rather than SO42- ions) which are oxidised to evolve O2 gas.
From the above discussion, it follows that during electrolysis of an aqueous solution of H2S04 only the electrolysis of H2O occurs liberating H2 at the cathode and O2 at the anode.
(iv) In aqueous solution, CuCl2 ionises as follows:
CuCl2(aq) ——-> CU2+(aq) + 2Cl–(aq)
On passing electricity, CU2+(aq) ions move towards cathode and CU2+(aq) ions move towards anode.
Thus, at cathode, either CU2+(aq) or H2O molecules are reduced. Their electrode potentials are:
CU2++ 2e– ———> Cu(s); E° = +0.34 V
H2O(l) + 2e– ——–> H2(g) + 2OH–; E° = -0.83 V
Since the electrode potential of CU2+(aq) ions is much higher than that of H2O, therefore, at the cathode, it is CU2+(aq) ions which are reduced and not H2Omolecules.
Similarly, at the anode, either Cl–(aq) ions or H2O molecules are oxidised. Their oxidation potentials
2Cl–(aq) ——> Cl2(g) + 2e–; AE° = -1.36 V
2H2O(l) ——>O2(g) + 4H+(aq) + 4e–; ∆E° = -1.23 V
Although oxidation potential of H2O molecules is higher than that of Cl– ions, nevertheless, oxidation of Cl–(aq) ions occurs in preference to H2O since due to overvoltage much lower potential than -1.36 V is needed for the oxidation of H2O molecules.
Thus, when an aqueous solution of CuCl2 is electrolysed, Cu metal is liberated at the cathode while Cl2 gas is evolved at the anode.
Question 28. Arrange the following metals in the order in which they displace each other from the solution of their salts.Al, Cu, Fe, Mg and Zn.
Answer: It is based upon the relative positions of these metals in the activity series. The correct order is Mg, Al, Zn, Fe, Cu .
Question 29. Given the standard electrode potentials,
K+/K = -2.93 V, Ag+/Ag = 0.80 V, Hg2+/Hg = 0.79 V, Mg2+/Mg = -2.37 V,
Cr3+/Cr = -0.74 V. Arrange these metals in increasing order of their reducing power.
Answer: Lower the electrode potential, better is the reducing agent. Since the electrode potentials increase in the oder; K+/K (-2.93 V), Mg2+/Mg (-2.37 V), Cr3+/Cr (-0.74 V), Hg2+/Hg (0.79 V), Ag+/Ag (0.80 V), therefore, reducing power of metals decreases in the same order, i.e., K, Mg, Cr, Hg, Ag.
latest video
news via inbox
Nulla turp dis cursus. Integer liberos euismod pretium faucibua