NCERT Solutions for Class 11 Chemistry Chapter 4 Chemical Bonding and Molecular Structure
NCERT Solutions for Class 11 Chemistry Chapter 4
Students willing to learn about the different concepts presented in the chapter can refer to the NCERT Solutions for Class 11 Chemistry Chapter 4 Chemical Bonding and Molecular Structure provided below. Our subject experts have presented detailed explanations of all the sections the chapter contains. Students can get in-depth knowledge and solve all the exercises of textbooks using the NCERT solutions for Class 11 Chemistry. These solutions are updated according to the latest syllabus prescribed by CBSE.
The NCERT Solutions for Class 11 Chemistry is extremely crucial study material to boost students’ confidence and prepare them for the next year’s board examinations as well as various entrance examinations. Students can view the solutions of each question by scrolling below.
Access the NCERT Solutions for Class 11 Chemistry Chapter 4 Chemical Bonding and Molecular Structure
Question 1. Explain the formation of a chemical bond.
Answer: According to Kossel and Lewis, atoms combine together in order to complete their respective octets so as to acquire the stable inert gas configuration. This can occur in two ways; by transfer of one or more electrons from one atom to other or by sharing of electrons between two or more atoms.
Question 2.Write Lewis dot symbols for atoms of the following elements: Mg, Na, B, O, N, Br.
Answer:

Question 3. {(i) Sulfur (S) and Sulfide Ion (S\(^2-\))} Sulfur (S) has 6 valence electrons. Its Lewis dot symbol is: \[ \text{S} \cdots \] \[ \text{S} : \cdots \] The sulfide ion (S\(^2-\)) has gained 2 additional electrons, making a total of 8 valence electrons.
The Lewis dot symbol for S\(^2-\) is: \[ [\text{S} \cdots \cdots]^{2-} \] \subsection*{(ii) Aluminum (Al) and Aluminum Ion (Al\(^3+\))} Aluminum (Al) has 3 valence electrons. Its Lewis dot symbol is: \[ \text{Al} \cdots \] \[ \text{Al} \cdot \cdot \cdot \] The aluminum ion (Al\(^3+\)) has lost its 3 valence electrons, so its Lewis dot symbol is: \[ [\text{Al}]^{3+} \] \subsection*{(iii) Hydrogen (H) and Hydride Ion (H\(^-\))} Hydrogen (H) has 1 valence electron. Its Lewis dot symbol is: \[ \text{H} \cdot \] The hydride ion (H\(^-\)) has gained 1 additional electron, making a total of 2 electrons. The Lewis dot symbol for H\(^-\) is: \[ [\text{H} \cdots]^{-} \]
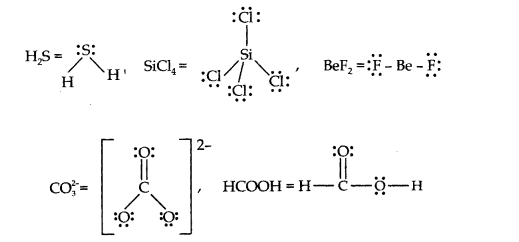
Question 4. Draw the Lewis structures for the following molecules and ions:
H2S, SiCl4 , BeF2, C032-, HCOOH
Answer:
Question 5. Define Octet rule. Write its significance and limitations.
Answer: Octet rule: Atoms of elements combine with each other in order to complete their respective octets so as to acquire the stable gas configuration.
Significance: It helps to explain why different atoms combine with each other to form ionic compounds or covalent compounds.
Limitations of Octet rule:
- According to Octet rule, atoms take part in chemical combination to achieve the configuration of nearest noble gas elements. However, some of noble gas elements like Xenon have formed compounds with fluorine and oxygen. For example: XeF2, XeF4 etc.
Therefore, validity of the octet rule has been challenged. - This theory does not account for shape of molecules.
Question 6. Write the favourable factors for the formation of ionic bond.
Answer:
- Low ionization enthalpy of metal atoms
- High electron gain enthalpy of non-metal atoms
- High lattice enthalpy of compound formed.
Question 7. Determine the shape of the following molecules using the VSEPR model: \begin{itemize} \item BeCl$_2$ \item BCl$_3$ \item SiCl$_4$ \item AsF$_5$ \item H$_2$S \item PH$_3$ \end{itemize} \subsection*
{Solution} \begin{tabular}{|l|c|c|c|} \hline \textbf{Molecule} & \textbf{Number of Electron Pairs Around Central Atom} & \textbf{Molecular Geometry} & \textbf{Bond Angles} \\ \hline BeCl$_2$ & 2 & Linear & 180$^\circ$ \\ BCl$_3$ & 3 & Trigonal Planar & 120$^\circ$ \\ SiCl$_4$ & 4 & Tetrahedral & 109.5$^\circ$ \\ AsF$_5$ & 5 & Trigonal Bipyramidal & Three 120$^\circ$, Two 90$^\circ$ \\ H$_2$S & 4 & Non-linear/Bent & Approximately 92$^\circ$ \\ PH$_3$ & 4 & Trigonal Pyramidal & Approximately 93.5$^\circ$ \\ \hline \end{tabular}
Question 8. Although geometries of NH3 and H20 molecules are distorted tetrahedral, bond angle in water is less than that of ammonia. Discuss.
Answer:
Because of two lone pairs of electrons on O-atom, repulsion on bond pairs is greater in H20 in comparison to NH3 . Thus, the bond angle is less in H20 molecules.
Question 9. How do you express the bond strength in terms of bond order?
Answer: Bond strength is directly proportional to the bond order. Greater the bond order, more is the bond strength.
Question 10. Define the bond-length.
Answer: Bond-length: It is the equilibrium distance between the nuclei of two bonded atoms in a molecule. Bond-lengths are measured by spectroscopic methods.
Question 11. Explain the important aspects of resonance with reference to the CO$_3^{2-}$ ion. \section*
{Solution} In the carbonate ion (CO$_3^{2-}$), resonance is an important concept that helps describe the delocalization of electrons. Here are the key aspects: \begin{itemize} \item \textbf{Delocalization of Electrons}: The pi bond electrons in the carbonate ion are delocalized over the three oxygen atoms. This delocalization means that the electrons are not confined to a single bond but are spread out over multiple bonds. \item \textbf{Resonance Structures}: The carbonate ion cannot be accurately represented by a single Lewis structure. Instead, it is described as a resonance hybrid of three equivalent structures. Each resonance structure shows different arrangements of double bonds and single bonds between the carbon and oxygen atoms. The resonance structures are: \begin{align*} \text{Structure 1:} & \quad \text{O} = \text{C} – \text{O}^- \text{ (single bond)} \text{ (double bond)} \text{O}^- \\ \text{Structure 2:} & \quad \text{O}^- \text{ = } \text{C} – \text{O} \text{ (single bond)} \text{ (double bond)} \text{O}^- \\ \text{Structure 3:} & \quad \text{O}^- \text{ (single bond)} \text{ = C} – \text{O} \text{ (double bond)} \text{O}^- \\ \end{align*} \item \textbf{Equivalent Bonds}: Due to resonance, all the carbon-oxygen bonds in the CO$_3^{2-}$ ion are equivalent. This means that each bond has a bond order of 1.33, which is an average of the single and double bonds depicted in the resonance structures. \item \textbf{Resonance Hybrid}: The actual structure of the carbonate ion is a resonance hybrid of these three structures. It reflects the equal bond lengths and strengths observed experimentally, which are intermediate between a single and a double bond. \end{itemize} Therefore, the carbonate ion (CO$_3^{2-}$) is best described by a resonance hybrid rather than a single Lewis structure.
Question 12. H3PO3 can be represented by structures 1 and 2 shown below. Can these two structures be taken as the canonical forms of the resonance hybrid representing H3PO3? If not, give reasons for the same.
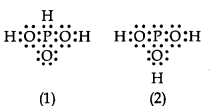
Answer: No, these cannot be taken as canonical forms because the positions of atoms have been changed.
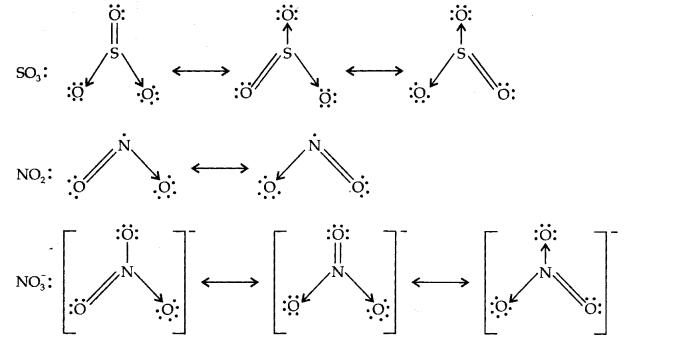
Question 13. Write the resonance structures for SO3,NO2 and NO3
Answer:
Question 14. Use Lewis symbols to show electron transfer between the following atoms to form cations and anions (a) K and S (b) Ca and O (c) Al and N.
Answer:
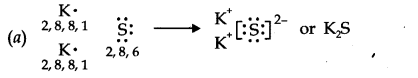
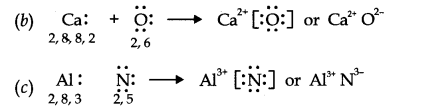
Question 15. Although both CO2 and H2O are triatomic molecules, the shape of H2O molecule is bent while that of CO2 is linear. Explain this on the basis of dipole moment.
Answer: In CO2, there are two C=O bonds. Each C=O bond is a polar bond.
The net dipole moment of CO2 molecule is zero. This is possible only if CO2 is a linear molecule. (O=C=O). The bond dipoles of two C=O bonds cancel the moment of each other.
Whereas, H2O molecule has a net dipole moment (1.84 D). H2O molecule has a bent structure because here the O—H bonds are oriented at an angle of 104.5° and do not cancel the bond moments of each other.
Question 16. Write the significance/applications of dipole moment.
Answer:
- In predicting the nature of the molecules: Molecules with specific dipole moments are polar in nature and those of zero dipole moments are non-polar in nature.
- In the determination of shapes of molecules.
- In calculating the percentage ionic character.
Question 17. Define electronegativity. How does it differ from electron gain enthalpy?
Answer: Electronegativity: Electronegativity is the tendency of an atom to attract shared pair of electrons. It is the property of bonded atom.
Whereas, electron gain enthalpy is the tendency of an atom to attract outside electron. It is the property of an isolated atom.
Question 18. Explain with the help of suitable example polar covalent bond.
Answer: When two atoms with different electronegativity are linked to each other by covalent bond, the shared electron pair will not in the centre because of the difference in electronegativity. For example, in hydrogen flouride molecule, flouride has greater electronegativity than hydrogen. Thus, the shared electron pair is displaced more towards’flourine atom, the later will acquire a partial negative charge (∂–). At the same time hydrogen atom will have a partial positive charge (∂+). Such a covalent bond is known as polar covalent bond or simply polar bond.
It is represented as
Question 19. Arrange the bonds in order of increasing ionic character in the molecules: LiF, K2O, N2, SO2 and ClF3.
Answer: N2 < SO2 < ClF3 < K2O < LiF
Question 20. The skeletal structure of CH3COOH as shown below is correct, but some of the bonds are shown incorrectly. Write the correct Lewis structure for acetic acid.
Answer:
Question 21. Apart from tetrahedral geometry, another possible geometry for CH4 is square planar with the four H atoms at the comers of the square and the C atom at its centre. Explain why CH4 is not square planar?
Answer: According to VSEPR theory, if CH4were square planar, the bond angle would be 90°. For tetrahedral structure, the bond angle is 109°28′. Therefore, in square planar structure, repulsion between bond pairs would be more and thus the stability will be less.
Question 22. Explain why BeH2 molecule has a zero dipole moment although the Be—H bonds are polar.
Answer: BeH2is a linear molecular (H—Be—H), the bond angle = 180°.
Be—H bonds are polar due to difference in their electronegativity but the bond polarities cancel each other. Thus, molecule has resultant dipole moment of zero.
Question 23. Which out of NH3 and NF3 has higher dipole moment and why?
Answer: In NH3 and NF3, the difference in electronegativity is nearly same but the dipole moment of NH3 = (1.46D) For Example, NH3 = (0.24D)
In NH3, the dipole moments of the three N—H bonds are in the same direction as the lone pair of electron. But in NF3, the dipole moments of the three N—F bonds are in the direction opposite to that of the lone pair. Therefore, the resultant dipole moment in NH3 is more than in NF3.
Question 24. What is meant by hybridisation of atomic orbitals? Describe the shapes of sp, sp2, sp3 hybrid orbitals.
Answer: Hybridisation: It is defined as the process of intermixing of atomic oribitals of slightly different energies to give rise to new hybridized orbitals having equivalent energy and identical shapes.
Shapes of Orbitals:
sp hybridisation: When one s-and one p-orbital, intermix then it is called sp-hybridisation. For example, in BeF2, Be atom undergoes sp-hybridisation. It has linear shape. Bond angle is 180°.
sp2 hybridisation: One s-and two p-orbitals get hybridised to form three equivalent hybrid orbitals. The three hybrid orbitals directed towards three corners of an equilateral triangle. It is, therefore, known as trigonal hybridisation.
sp3 hybridisation: One s-and three p-orbitals get hybridised to form four equivalent hybrid orbitals. These orbitals are directed towards the four corners of a regular tetrahedron.
Question 25. Describe the change in hybridisation (if any) of the Al atom in the following reaction. AlCl3 + Cl– ——> AlCl4- .
Answer: Electronic configuration of 13Al = 1s2 2s2 2p6 3s1 3px13py1
(excited state)
Hence, hybridisation will be SP2
In AlCl–4, the empty 3pz orbital is also involved. So, the hybridisation is sp3 and the shape is tetrahedral.
Question 26. Is there any change in the hybridisation ofB and N atoms as a result of the following reaction ? BF3 + NH3 ——-> F3 B.NH3
Answer: In BF3, B atom is sp2 hybridised. In NH3, N is sp3 hybridised.
After the reaction, hybridisation of B changes from sp2 to sp3.
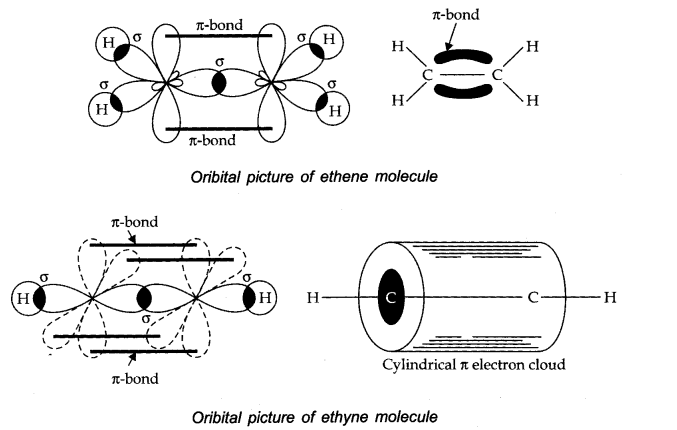
Question 27. Draw diagrams showing the formation of a double bond and a triple bond between carbon atoms in C2 H4 and C2 H2 molecules.
Answer:
Ques 28: How many sigma and pi bonds are present in: \begin{itemize} \item (a) \(\text{C}_2\text{H}_2\) \item (b) \(\text{C}_2\text{H}_4\) \end{itemize} \section*
{Solution} \begin{itemize} \item \textbf{For } \(\text{C}_2\text{H}_2\): The structure of \(\text{C}_2\text{H}_2\) is: \[ \text{H} – \text{C} \equiv \text{C} – \text{H} \] – \textbf{Sigma Bonds:} There are 3 sigma bonds. Each hydrogen-carbon bond and the carbon-carbon single bond are sigma bonds. – \textbf{Pi Bonds:} There are 2 pi bonds in the carbon-carbon triple bond. Thus, \(\text{C}_2\text{H}_2\) has 3 sigma bonds and 2 pi bonds. \item \textbf{For } \(\text{C}_2\text{H}_4\): The structure of \(\text{C}_2\text{H}_4\) is: \[ \text{H}_2 \text{C} = \text{C} \text{H}_2 \] – \textbf{Sigma Bonds:} There are 5 sigma bonds. Each hydrogen-carbon bond and the carbon-carbon single bond are sigma bonds. – \textbf{Pi Bonds:} There is 1 pi bond in the carbon-carbon double bond. Thus, \(\text{C}_2\text{H}_4\) has 5 sigma bonds and 1 pi bond.
Question 29. Considering X-axis as the intemuclear axis which out of the following will not form a sigma bond and why? (a) Is and Is (b) Is and 2px (c) 2py and 2py (d) Is and 2s
Answer: (c) It will not form a s-bond because taking x-axis as the intemuclear axis, there will be lateral overlap between the two 2py orbitals forming a Π -bond.
Question 30. Which hybrid orbitals are used by carbon atoms in the following molecules?
(a) CH3-CH3 (b) CH3-CH = CH2 (c) CH3-CH2-OH (d) CH3-CHO (e) CH3COOH.
Answer:
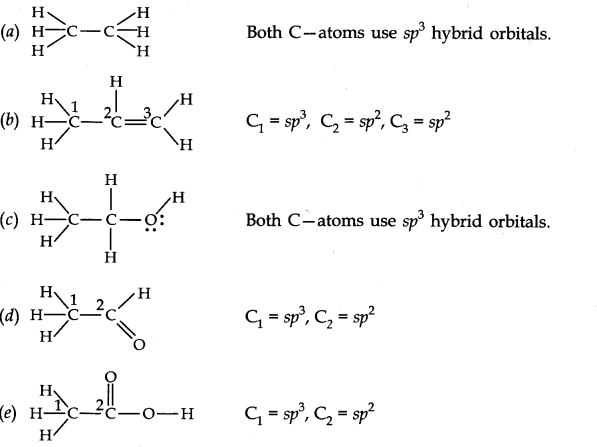
Question 31. What do you understand by bond pairs and lone pairs of electrons? Illustrate by giving one example of each type. \section*
{Solution} When two atoms combine by sharing their one or more valence electrons, a covalent bond is formed between them. The shared pairs of electrons present between the bonded atoms are called \textbf{bond pairs}. All valence electrons may not participate in bonding. The electron pairs that do not participate in bonding are called \textbf{lone pairs of electrons}. \begin{itemize} \item \textbf{Example of Bond Pairs:} In \(\text{C}_2\text{H}_6\) (ethane), each carbon atom forms a single bond with three hydrogen atoms and a single bond with the other carbon atom. Here, there are seven bond pairs in total, and no lone pairs are present on the carbon atoms. \[ \text{H}-\text{C}-\text{C}-\text{H} \] \item \textbf{Example of Lone Pairs:} In \(\text{H}_2\text{O}\) (water), the central oxygen atom forms two single bonds with two hydrogen atoms. The oxygen atom also has two lone pairs of electrons. \[ \text{H}-\text{O}-\text{H} \] The oxygen atom’s electron configuration includes four pairs of electrons: two bond pairs (one for each \(\text{O-H}\) bond) and two lone pairs. \end{itemize}
Question 32. Distinguish between a sigma bond and a pi bond.
Answer:

Question 33. Explain the formation of H2 molecule on the basis of valence bond theory.
Answer: Let us consider the combination between atoms of hydrogen HA and HB and eA and eB be their respective electrons.
As they tend to come closer, two different forces operate between the nucleus and the electron of the other and vice versa. The nuclei of the atoms as well as their electrons repel each other. Energy is needed to overcome the force of repulsion. Although the number of new attractive and repulsive forces is the same, but the magnitude of the attractive forces is more. Thus, when two hydrogen atoms approach each other, the overall potential energy of the system decreases. Thus, a stable molecule of hydrogen is formed.
Question 34. Write the important conditions required for the linear combination of atomic orbitals to form molecular orbitals.
Answer:
- The combining atomic orbitals should have comparable energies.
For example, Is orbital of one atom can combine with Is atomic orbital of another atom, 2s can combine with 2s. - The combining atomic orbitals must have proper orientations. So that they are able to overlap to a considerable extent.
- The extent of overlapping should be large.
Question 35. Use molecular orbital theory to explain why the Be2 molecule does not exist.
Answer:
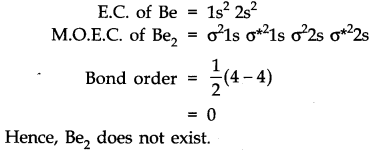
Question 36. Compare the relative stability of the following species and indicate their magnetic properties: O2, O2, O2– (Superoxide),O22- (peroxide)
Answer: O2— Bond order = 2, paramagnetic
O2+— Bond order = 2.5, paramagnetic
O2–— Bond order = 1.5, paramagnetic
O22- — Bond order = 1, diamagnetic
Order of relative stability is
O2+ > O2 > O2– > O22-
(2.5) (2.0) (1.5) (1.0)
Question 37. Write the significance of plus and minus sign in representing the orbitals,
Answer: Plus and minus sign is used to indentify the nature of electrons wave. Plus (+ve) sign denotes crest, while (-ve) sign denotes trough.
Question 38. Describe the hybridisation in case of \(\text{PCl}_5\). Why are the axial bonds longer as compared to equatorial bonds? \section*
{Solution} Phosphorus (P) has the ground state valence shell electronic configuration: \[ 3s^2 \, 3p^3 \] In the excited state, the electronic configuration is: \[ 3s^1 \, 3p^3 \, 3d^1 \] Phosphorus undergoes \( \text{sp}^3\text{d} \) hybridization. This hybridization involves the mixing of one \(3s\), three \(3p\), and one \(3d\) orbitals to form five equivalent \( \text{sp}^3\text{d} \) hybrid orbitals. The hybridized orbitals arrange themselves in a trigonal bipyramidal geometry. In this geometry: – Three \( \text{Cl} \) atoms occupy the equatorial plane, forming \(120^\circ\) angles with each other. – Two \( \text{Cl} \) atoms occupy the axial positions, forming \(90^\circ\) angles with the equatorial plane. The axial bonds are longer compared to the equatorial bonds due to increased electron repulsion. This is because: – The axial bonds are subjected to greater repulsion from the lone pairs in the equatorial plane. – The equatorial bonds experience less repulsion since they are positioned at a greater distance from each other compared to the axial bonds. Hence, the axial bonds are longer compared to the equatorial bonds.
Question 39. Define hydrogen bonds. Is it weaker or stronger than the van der Waals forces?
Answer: When hydrogen is attached with highly electronegative element in a covalent bonding the electrons of the covalent bond are shifted towards the more electronegative atom. Thus, a partially positively charged hydrogen atom forms a bond with the other more electronegative atom. This bond is known as a hydrogen bond. Hydrogen bond is stronger than the van der Waals forces.
Question 40. What is meant by the term bond order? Calculate the bond order of \(\text{N}_2\), \(\text{O}_2\), \(\text{O}_2^+\), and \(\text{O}_2^-\). \section*
{Solution} The bond order is a measure of the number of covalent bonds present in a molecule. It is defined as half the difference between the number of electrons in the bonding molecular orbitals and the number of electrons in the antibonding molecular orbitals. Mathematically, it is given by: \[ \text{Bond Order} = \frac{N_b – N_{ab}}{2} \] where \( N_b \) is the number of electrons in bonding molecular orbitals, and \( N_{ab} \) is the number of electrons in antibonding molecular orbitals. \begin{enumerate} \item \textbf{For \(\text{N}_2\) molecule:} The electronic configuration is: \[ \text{K} \text{K} (\sigma_{2s})^2 (\sigma^*_{2s})^2 (\pi_{2p_x})^2 (\pi_{2p_y})^2 (\sigma_{2p_z})^2 \] Number of electrons in bonding molecular orbitals = \(8\) \\ Number of electrons in antibonding molecular orbitals = \(2\) \\ Bond order: \[ \text{Bond Order} = \frac{8 – 2}{2} = 3 \] \item \textbf{For \(\text{O}_2\) molecule:} The electronic configuration is: \[ \text{K} \text{K} (\sigma_{2s})^2 (\sigma^*_{2s})^2 (\sigma_{2p_z})^2 (\pi_{2p_x})^2 (\pi_{2p_y})^2 (\pi^*_{2p_x})^1 (\pi^*_{2p_y})^1 \] Number of electrons in bonding molecular orbitals = \(8\) \\ Number of electrons in antibonding molecular orbitals = \(4\) \\ Bond order: \[ \text{Bond Order} = \frac{8 – 4}{2} = 2 \] \item \textbf{For \(\text{O}_2^+\) ion:} The electronic configuration is: \[ \text{K} \text{K} (\sigma_{2s})^2 (\sigma^*_{2s})^2 (\sigma_{2p_z})^2 (\pi_{2p_x})^2 (\pi_{2p_y})^2 (\pi^*_{2p_x})^1 \] Number of electrons in bonding molecular orbitals = \(8\) \\ Number of electrons in antibonding molecular orbitals = \(3\) \\ Bond order: \[ \text{Bond Order} = \frac{8 – 3}{2} = 2.5 \] \item \textbf{For \(\text{O}_2^-\) ion:} The electronic configuration is: \[ \text{K} \text{K} (\sigma_{2s})^2 (\sigma^*_{2s})^2 (\sigma_{2p_z})^2 (\pi_{2p_x})^2 (\pi_{2p_y})^2 (\pi^*_{2p_x})^2 (\pi^*_{2p_y})^1 \] Number of electrons in bonding molecular orbitals = \(8\) \\ Number of electrons in antibonding molecular orbitals = \(5\) \\ Bond order: \[ \text{Bond Order} = \frac{8 – 5}{2} = 1.5 \]
latest video
news via inbox
Nulla turp dis cursus. Integer liberos euismod pretium faucibua