NCERT Exemplar Class 11 Chemistry Chapter 10 The S Block Elements
NCERT Exemplar for Class 11 Chemistry Chapter 10
Students can access the Solutions of NCERT Exemplar for Class 11 Chemistry Chapter 10 provided below by the top subject-matter experts at SimplyAcad. As we know, Chemistry is a challenging yet interesting subject, students must revise all the portions of each chapter carefully and time-to-time. Through the given solutions of Chapter 10 The S Block Element, learners will gain in-depth understanding and analysis of important questions that can be formed.
NCERT Exemplar for Class 11 Chemistry is a crucial resource for all the students preparing for next year’s board or medical entrances to build the base strong. Students will be more familiarised by different question patterns such as MCQs (two types), short answer, matching and assertion and reason type questions. The provided exemplar contains a total of 45 important questions about the elements which are present in the S block, such as hydrogen, helium, lithium, beryllium, sodium etc. Access and learn from the given solution set to perform well in your coming exams.
Access the NCERT Exemplar Class 11 Chemistry Chapter 10 The S Block Element
I. Multiple Choice Questions (Type-I)
1. The alkali metals are low melting. Which of the following alkali metal is expected
to melt if the room temperature rises to 30°C?
(i) Na
(ii) K
(iii) Rb
(iv) Cs
Solution:
Option (iv) is the answer.
2. Alkali metals react with water vigorously to form hydroxides and dihydrogen.
Which of the following alkali metals reacts with water least vigorously?
(i) Li
(ii) Na
(iii) K
(iv) Cs
Solution:
Option (i) is the answer.
3. The reducing power of a metal depends on various factors. Suggest the factor
which makes Li, the strongest reducing agent in aqueous solution.
(i) Sublimation enthalpy
(ii) Ionisation enthalpy
(iii) Hydration enthalpy
(iv) Electron-gain enthalpy
Solution:
Option (iii) is the answer.
4. Metal carbonates decompose on heating to give metal oxide and carbon
dioxide. Which of the metal carbonates is most stable thermally?
(i) MgCO3
(ii) CaCO3
(iii) SrCO3
(iv) BaCO3
Solution:
Option (iv) is the answer.
5. Which of the carbonates given below is unstable in air and is kept in CO2
atmosphere to avoid decomposition.
(i) BeCO3
(ii) MgCO3
(iii) CaCO3
(iv) BaCO3
Solution:
Option (i) is the answer.
6. Metals form basic hydroxides. Which of the following metal hydroxide is the
least basic?
(i) Mg(OH)2
(ii) Ca(OH)2
(iii) Sr(OH)2
(iv) Ba(OH)2
Solution:
Option (i) is the answer
7. Some of the Group 2 metal halides are covalent and soluble in organic solvents.
Among the following metal halides, the one which is soluble in ethanol is
(i) BeCl2
(ii) MgCl2
(iii) CaCl2
(iv) SrCl2
Solution:
Option (i) is the answer.
8. The order of decreasing ionisation enthalpy in alkali metals is
(i) Na > Li > K > Rb
(ii) Rb < Na < K < Li
(iii) Li > Na > K > Rb
(iv) K < Li < Na < Rb
Solution:
Option (iii) is the answer.
9. The solubility of metal halides depends on their nature, lattice enthalpy and
hydration enthalpy of the individual ions. Amongst fluorides of alkali metals,
the lowest solubility of LiF in water is due to
(i) Ionic nature of lithium fluoride
(ii) High lattice enthalpy
(iii) High hydration enthalpy for lithium-ion.
(iv) Low ionisation enthalpy of the lithium atom
Solution:
Option (ii) is the answer.
10. Amphoteric hydroxides react with both alkalies and acids. Which of the
following Group 2 metal hydroxides is soluble in sodium hydroxide?
(i) Be(OH)2
(ii) Mg(OH)2
(iii) Ca(OH)2
(iv) Ba(OH)2
Solution:
Option (i) is the answer.
11. In the synthesis of sodium carbonate, the recovery of ammonia is done by
treating NH4Cl with Ca(OH)2. The by-product obtained in this process is
(i) CaCl2
(ii) NaCl
(iii) NaOH
(iv) NaHCO3
Solution:
Option (i) is the answer.
12. When sodium is dissolved in liquid ammonia, a solution of deep blue colour
is obtained. The colour of the solution is due to
(i) ammoniated electron
(ii) sodium ion
(iii) sodium amide
(iv) ammoniated sodium ion
Solution:
Option (i) is the answer.
13. By adding gypsum to cement
(i) setting time of cement becomes less.
(ii) setting time of cement increases.
(iii) colour of cement becomes light.
(iv) the shining surface is obtained.
Solution:
Option (ii) is the answer.
14. Dead burnt plaster is
(i) CaSO4
(ii) CaSO4.1/2 H2O
(iii) CaSO4.H2O
(iv) CaSO4.2H2O
Solution:
Option (i) is the answer.
15. Suspension of slaked lime in water is known as
(i) lime water
(ii) quick lime
(iii) milk of lime
(iv) an aqueous solution of slaked limeSolution:
Option (iii) is the answer.
16. Which of the following elements does not form hydride by direct heating with
dihydrogen?
(i) Be
(ii) Mg
(iii) Sr
(iv) Ba
Solution:
Option (i) is the answer.
17. The formula of soda ash is
(i) Na2CO3.10H2O
(ii) Na2CO3.2H2O
(iii) Na2CO3.H2O
(iv) Na2CO3
Solution:
Option (iv) is the answer.
18. A substance which gives brick red flame and breaks down on heating to give
oxygen and a brown gas is
(i) Magnesium nitrate
(ii) Calcium nitrate
(iii) Barium nitrate
(iv) Strontium nitrate
Solution:
Option (ii) is the answer.
19. Which of the following statements is true about Ca(OH)2?
(i) It is used in the preparation of bleaching powder
(ii) It is a light blue solid
(iii) It does not possess disinfectant property.
(iv) It is used in the manufacture of cement.
Solution:
Option (i) is the answer.
20. A chemical A is used for the preparation of washing soda to recover ammonia.
When CO2 is bubbled through an aqueous solution of A, the solution turns
milky. It is used in whitewashing due to disinfectant nature. What is the
the chemical formula of A?
(i) Ca (HCO3)2
(ii) Cao
(iii) Ca(OH)2
(iv) CaCO3
Solution:
Option (iii) is the answer.
21. Dehydration of hydrates of halides of calcium, barium and strontium i.e.,
CaCl26H2O, BaCl2.2H2O, SrCl2.2H2O, can be achieved by heating. These
become wet on keeping in air. Which of the following statements is correct
about these halides?
(i) act as dehydrating agent
(ii) can absorb moisture from the air
(iii) The tendency to form hydrate decreases from calcium to barium
(iv) All of the above
Solution:
Option (iv) is the answer.
II. Multiple Choice Questions (Type-II)
In the following questions, two or more options may be correct.
22. Metallic elements are described by their standard electrode potential, fusion
enthalpy, atomic size, etc. The alkali metals are characterised by which of the
following properties?
(i) High boiling point
(ii) High negative standard electrode potential
(iii) High density
(iv) Large atomic size
Solution:
Option (ii) and (iv) are the answers.
23. Several sodium compounds find use in industries. Which of the following
compounds are used for textile industry?
(i) Na2CO3
(ii) NaHCO3
(iii) NaOH
(iv) NaCl
Solution:
Option (i) and (iii) are the answers.
24. Which of the following compounds are readily soluble in water?
(i) BeSO4
(ii) MgSO4
(iii) BaSO4
(iv) SrSO4
Solution:
Option (i) and (ii) are the answers.
25. When Zeolite, which is hydrated sodium aluminium silicate is treated with
hard water, the sodium ions are exchanged with which of the following ion(s)?
(i) H+ ions
(ii) Mg2+ ions
(iii) Ca2+ ions
(iv) SO42- ionsSolution:
Option (ii) and (iii) are the answers.
26. Identify the correct formula of halides of alkaline earth metals from the following.
(i) BaCl2.2H2O
(ii) BaCl2.4H2O
(iii) CaCl2.6H2O
(iv) SrCl2.4H2O
Solution:
Option (i) and (iii) are the answers.
27. Choose the correct statements from the following.
(i) Beryllium is not readily attacked by acids because of the presence of
an oxide film on the surface of the metal.
(ii) Beryllium sulphate is readily soluble in water as the greater hydration
enthalpy of Be2+ overcomes the lattice enthalpy factor.
(iii) Beryllium exhibits coordination number more than four.
(iv) Beryllium oxide is purely acidic.
Solution:
Option (i) and (ii) are the answers.
28. Which of the following are the correct reasons for the anomalous behaviour of lithium?
(i) The exceptionally small size of its atom
(ii) Its high polarising power
(iii) It has a high degree of hydration
(iv) Exceptionally low ionisation enthalpy
Solution:
Option (i) and (ii) are the answers.
III. Short Answer Type
29. How do you account for the strong reducing power of lithium in aqueous
solution?
Solution:
Lithium has the highest negative EӨ value, which is –3.04V. Lithium has a small atomic size, the highest ionization enthalpy but it is compensated by its high hydration enthalpy. Due to this, the reducing power of lithium is highest in an aqueous solution.
30. When heated in air, the alkali metals form various oxides. Mention the oxides formed by Li, Na and K.
Solution:
Lithium forms monoxide, sodium forms peroxide, and potassium form superoxide.
31. Complete the following reactions
(i) O2-2 + H2O →
(ii) O-2 + H2O →
Solution:
(i) O2-2 + 2H2O → H2O2 + 2OH-
(ii) O2– + 2H2O → H2O2 + O2 + 2OH-
32. Lithium resembles magnesium in some of its properties. Mention two such properties and give reasons for this resemblance.
Solution:
(i) Lithium and magnesium are both lighter and harder than the other metals in their respective groups.
(ii) Halides of both elements, LiCl and MgCl2 are soluble in ethanol.
These two elements have similar properties because of their similar atomic and ionic radii.
33. Name an element from Group 2 which forms an amphoteric oxide and a water-soluble sulphate.
Solution:
Beryllium oxide is amphoteric, unlike the other basic compounds.
34. Discuss the trend of the following:
(i) Thermal stability of carbonates of Group 2 elements.
(ii) The solubility and the nature of oxides of Group 2 elements.
Solution:
(i) The thermal stability of the carbonates increases with increasing cationic size. The more stable the oxide of an alkaline earth metal, the less stable is the carbonate of the same. Hence BeCO3 is highly unstable as BeO is stable.
(ii) Alkali metals form oxides with oxygen and give metal oxides. The oxides will be basic except BeO because BeO is amphoteric. hey also react with water to form sparingly soluble hydroxides. As the size of the cations increase BeO and MgO have the highest lattice energy and they are insoluble in water.
35. Why are BeSO4 and MgSO4 readily soluble in water while CaSO4, SrSO4 and BaSO4 are insoluble?
Solution:
BeSO4 and MgSO4 are readily soluble in water, CaSO4, SrSO4, and BaSO4 are insoluble. This is because the greater hydration enthalpies of Be2+ and Mg2+ ions overcome the lattice enthalpy factor and therefore their sulphate is soluble in water.
36. All compounds of alkali metals are easily soluble in water but lithium compounds are more soluble in organic solvents. Explain.
Solution:
The alkali metal compounds form ionic compounds due to their large ionic size and low ionization enthalpy, while lithium forms compounds of covalent nature due to their small ionic size, high ionization enthalpy, and high electronegativity.
37. In the Solvay process, can we obtain sodium carbonate directly by treating the solution containing (NH4) 2CO3 with sodium chloride? Explain.
Solution:
In the Solvay process, carbon dioxide is passed through a concentrated solution of sodium chloride saturated with ammonia, which forms ammonium carbonate followed by ammonium hydrogen carbonate. Ammonium hydrogen carbonate crystals separate and they are heated to form sodium carbonate. NH3 is recovered from the solution which contains NH4Cl is heated and treated with Ca(OH)2. The reaction of (NH4)2CO3 with NaCl gives two products, Na2CO3 and NH4Cl which are both soluble in water which does not shift the equilibrium to the right.
38. Write Lewis structure of O-2 ion and find out oxidation state of each oxygen atom? What is the average oxidation state of oxygen in this ion?
Solution:
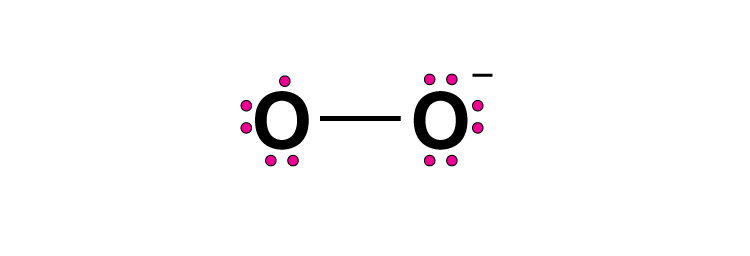
Oxygen atom with zero charges has 6 electrons, therefore the oxidation state is 0. When the oxygen atom has a negative charge, it has 7 electrons. Hence, the oxidation state is -1. The average oxidation state is 0 + (-1)/2 = -1/2.
39. Why do beryllium and magnesium not impart colour to the flame in the flame test?
Solution:
Be and Mg electrons are tightly bound to the atom due to the small atomic and ionic size. The flame is due to the excitation of the electron from its energy states. The electrons of Be and Mgdo did not gain excitation from the energy provided by the flame. Hence they do not show any flame in the flame test.
40. What is the structure of BeCl2 molecule in gaseous and solid-state?
Solution:
The gaseous/vapour state is different than the solid-state. The structure of BeCl2 in the solid-state is a polymeric chain structure. BeCl2 tends to form a chloro-bridged dimer at temperatures below 1200K and dissociates into a linear monomer at high temperatures of the order of 1200 K.
IV. Matching Type
In the following questions more than one option of column I and II maybe
correlated.
41. Match the elements given in Column I with the properties mentioned in Column II.
| Column I
(i) Li (ii) Na (iii) Ca (iv) Ba
|
Column II
(a)Insoluble sulphate (b)Strongest monoacidic base (c) Most negative E value among alkali metals. (d)Insoluble oxalate (e) 6s2 outer electronic configuration |
|---|
Solution:
(i) is c
(ii) is b
(iii) is d
(iv) is a,e
42. Match the compounds given in Column I with their uses mentioned in Column II.
| Column I
(i) CaCO3 (ii) Ca(OH)2 (iii) Cao (iv) CaSO4 |
Column II
(a) Dentistry, ornamental work (b) Manufacture of sodium carbonate from caustic soda (c) Manufacture of high-quality paper (d) Used in whitewashing |
|---|
Solution:
(i) is c
(ii) is d
(iii) is b
(iv) is a
43. Match the elements given in Column I with the colour they impart to the
flame has given in Column II.
| Column I
(i) Cs (ii) Na (iii) K (iv) Ca (v) Sr (vi) Ba |
Column II
(a) Apple green ((b) Violet (c) Brick red (d) Yellow (e) Crimson red (f) Blue |
|---|
Solution:
(i) is f
(ii) is d
(iii) is b
(iv) is c
(v) is e
(vi) is a
V. Assertion and Reason Type
In the following questions, a statement of Assertion (A) followed by a statement
of Reason (R) is given. Choose the correct option out of the choices given
below each question.
44. Assertion (A): The carbonate of lithium decomposes easily on heating to form lithium oxide and CO2.
Reason (R): Lithium being very small in size polarises large carbonate ion leading to the formation of more stable Li2O and CO2.
(i) Both A and R are correct and R is the correct explanation of A.
(ii) Both A and R are correct but R is not the correct explanation of A.
(iii) Both A and R are not correct
(iv) A is not correct but R is correct.
Solution:
Option (i) is correct.
45. Assertion (A): Beryllium carbonate is kept in the atmosphere of carbon dioxide.
Reason (R): Beryllium carbonate is unstable and decomposes to give beryllium oxide and carbon dioxide.
(i) Both A and R are correct and R is the correct explanation of A.
(ii) Both A and R are correct but R is not the correct explanation of A.
(iii) Both A and R are not correct.
(iv) A is not correct but R is correct.
Solution:
Option (i) is correct.
NCERT Exemplar For Class 11 Science
The NCERT exemplars are an effective study material for scoring higher marks in the examination paper. Students must practise these additional questions for their own benefits, as these are curated by the best subject-matter experts to boost both knowledge and confidence. Students can easily access the ncert exemplar for class 11 science by visiting our website SimplyAcad and solve all the questions listed to secure maximum marks.
Here are some other NCERT exemplar for class 11 chemistry:
| NCERT exemplar for class 11 chemistry Chapter 1 | NCERT exemplar for class 11 chemistry Chapter 7 |
|---|---|
| NCERT exemplar for class 11 chemistry Chapter 2 | NCERT exemplar for class 11 chemistry Chapter 8 |
| NCERT exemplar for class 11 chemistry Chapter 3 | NCERT exemplar for class 11 chemistry Chapter 9 |
| NCERT exemplar for class 11 chemistry Chapter 5 | NCERT exemplar for class 11 chemistry Chapter 11 |
| NCERT exemplar for class 11 chemistry Chapter 6 | NCERT exemplar for class 11 chemistry Chapter 12 |
latest video
news via inbox
Nulla turp dis cursus. Integer liberos euismod pretium faucibua