NCERT Exemplar Class 11 Chemistry Chapter 13 Hydrocarbons
NCERT Exemplar for Class 11 Chemistry Chapter 13: Hydrocarbons
Introduction to NCERT Exemplar for Class 11 Chemistry
SimplyAcad has provided the NCERT Exemplar for Class 11 Chemistry below to help students learn about the different topics in a detailed manner. The exemplar will allow students to gain deep insights of all the sections and prepares you better for the upcoming examination. Chemistry requires attention to minor details and information, therefore, solving exemplars will be an effective way to increase your marks.The given exemplar contains MCQs of two different types, Short Answer, Matching, Assertion and Reason, and Long Answer Type Questions, there are a total of 51 questions asked. Students can access the NCERT exemplar for class 11 chemistry, Chapter 13 Hydrocarbons by scrolling below. Along with this, there are several NCERT exemplar for class 11 science of all the chapters provided on this platform.
Access Solutions for NCERT Exemplar Class 11 Chemistry Chapter 13
Multiple Choice Questions (Type-I)
1. Arrange the following in decreasing order of their boiling points.
(A) n-butane (B) 2–methyl butane
(C) n-pentane (D) 2, 2–dimethylpropane
(i) A > B > C > D
(ii) B > C > D > A
(iii) D > C > B > A
(iv) C > B > D > A
Ans: (iv) C > B > D > A
The boiling point increases with the increase in the length of the side chain. However, with the branching, the boiling point decreases due to the decrease in the surface area.
2. Arrange the halogens F2, Cl2, Br2, I2, in order of their increasing reactivity with alkanes.
(i) I2< Br2< Cl2< F2
(ii) Br2< Cl2< F2< I2
(iii) F2< Cl2< Br2< I2
(iv) Br2< I2 < Cl2< F2
Ans: (i) I2< Br2< Cl2< F2
The reactivity of halogens with alkanes depends on the electronegativity i.e. higher the electronegativity higher would be the reaction. As down the group reactivity decrease so thus its reactivity.
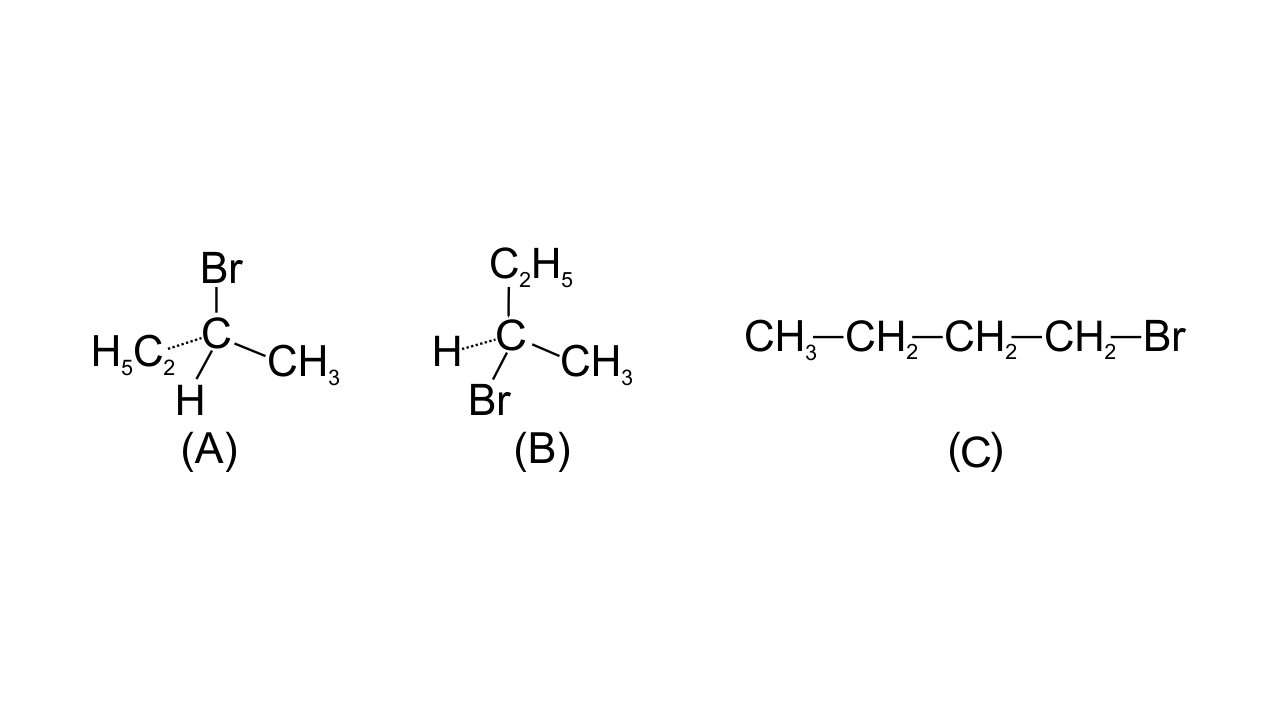
3. The increasing order of reduction of alkyl halides with zinc and dilute HCl is
(i) R–Cl< R–I < R–Br
(ii) R–Cl< R–Br < R–I
(iii) R–I < R–Br < R–Cl
(iv) R–Br< R–I < R–Cl
Ans: (ii) R–Cl< R–Br < R–I
The reduction of alkyl halides depends upon the C-X bond strength i.e. lesser the bond strength higher will be the reactivity of alkyl halide. As down the group size of the halogens increases thus the bond strength decreases.
4. The correct IUPAC name of the following alkane is
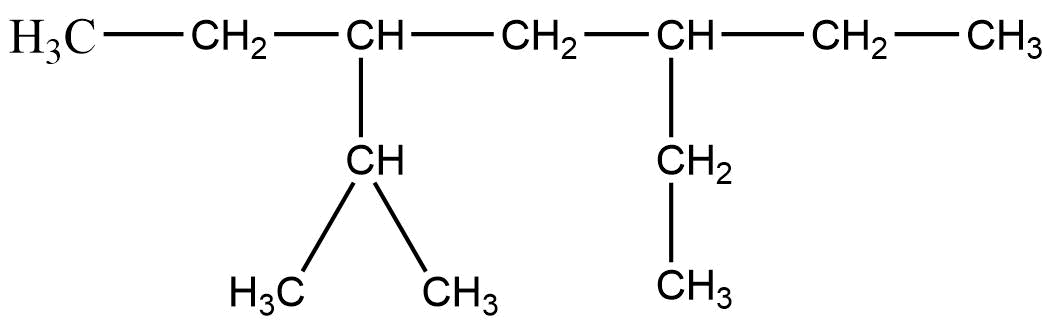
(i) 3, 6 – Diethyl – 2 – methyl octane
(ii) 5 – Isopropyl – 3 – ethyl octane
(iii) 3 – Ethyl – 5 – isopropyl octane
(iv) 3 – Isopropyl – 6 – ethyl octane
Ans: (i) 3, 6 – Diethyl – 2 – methyl octane
Octane is the longest chain here and the side chain follows the lowest sum rule.
5. The addition of HBr to 1-butene gives a mixture of products A, B and C
The mixture consists of
(i) A and B as major and C as minor products
(ii) B as major, A and C as minor products
(iii) B as minor, A and C as major products
(iv) A and B as minor and C as major products
Ans: (i)
The reaction follows Markovnikov’s rule leading to giving 2o carbocation as an intermediate.
6. Which of the following will not show geometrical isomerism?
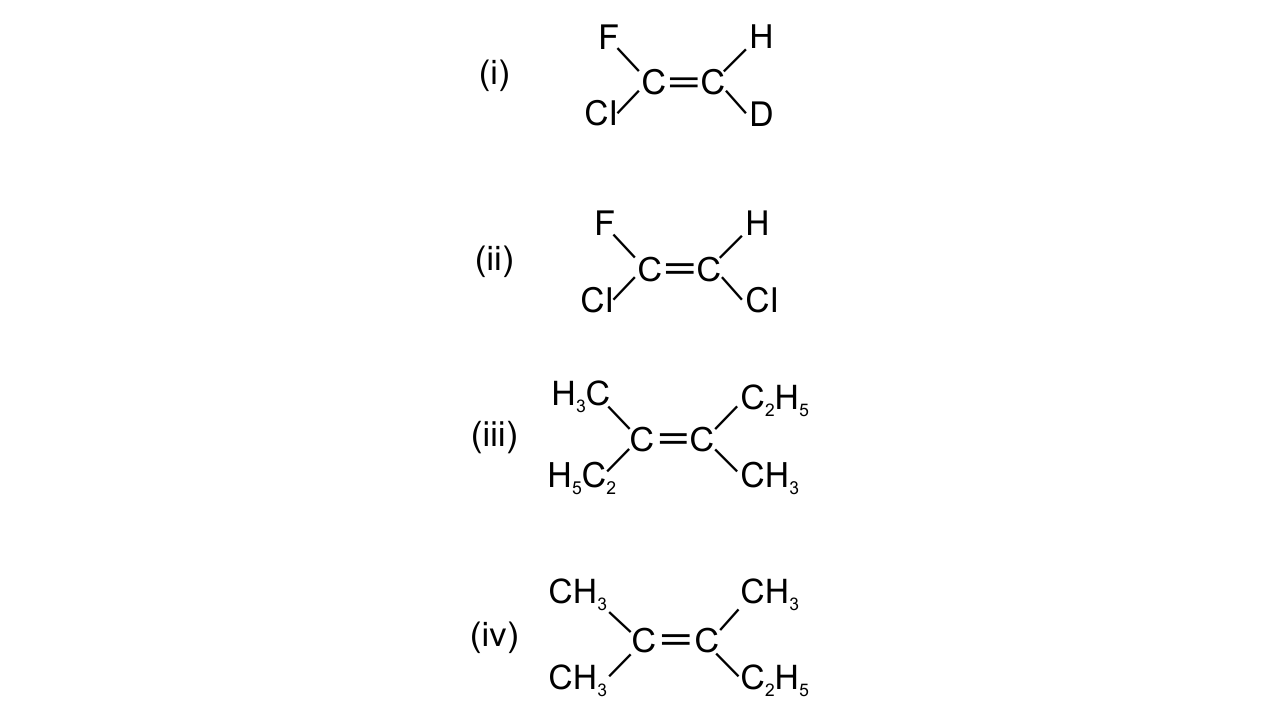
Ans: (iv)
The two substituents attached to the same C atom of the C=C bond should be different to exhibit geometrical isomerisms.
7. Arrange the following hydrogen halides in order of their decreasing reactivity with propene.
(i) HCl>HBr> HI
(ii) HBr> HI >HCl
(iii) HI >HBr>HCl
(iv) HCl> HI >HBr
Ans: (iii)
The addition of alkyl halides to propene depends upon the H-X bond strength i.e. lesser the bond strength higher will be the reactivity of the alkyl halide. As down the group size of the halogens increases thus the bond strength decreases.
8. Arrange the following carbanions in order of their decreasing stability.
(A) H3C – C ≡ C–
(B) H – C ≡ C–
(C) H3C – CH2
(i) A > B > C
(ii) B > A > C
(iii) C > B > A
(iv) C > A > B
Ans: (ii)
The electronegativity of sp hybridization is higher than that of sp3 hybridization.
The +I effect destabilizes the carbanion.
9. Arrange the following alkyl halides in decreasing order of the rate of β– elimination reaction with alcoholic KOH.
(i) A > B > C
(ii) C > B > A
(iii) B > C > A
(iv) A> C > B
Ans: (iv)
The order of reactivity of β– elimination reaction is 3o>2o>1o
10. Which of the following reactions of methane is incomplete combustion:
(i) 2CH4 + O2
−
→
−
−
−
−
−
−
−
−
Cu/523k/100atm
→Cu/523k/100atm
2CH3OH
(ii) CH4 + O2
−
→
−
−
M
2
0
3
→M203
HCHO + H2O
(iii) CH4 + O2 → C (s) + 2H2O (l)
(iv) CH4 + O2 → CO2 (g) + 2H2O (l)
Ans: (iii)
As the unburn carbon left out in this reaction thus it is an incomplete reaction
Multiple Choice Questions (Type-II)
11. Some oxidation reactions of methane are given below. Which of them is/are controlled oxidation reactions?
(i) CH4 + O2→ CO2 (g) + 2H2O (l)
(ii) CH4 + O2→ C (s) + 2H2O (l)
(iii) CH4 + O2
−
→
−
−
M
2
0
3
→M203
HCHO + H2O
(iv) 2CH4 + O2
−
→
−
−
−
−
−
−
−
−
Cu/523k/100atm
→Cu/523k/100atm
2CH3OH
Ans: (iii) & (iv)
Methane is a controlled oxidation reaction that leads to giving formaldehyde and methanol.
12. Which of the following alkenes on ozonolysis give a mixture of ketones only?

Ans: (iii) & (iv)
When only the alkyl group is attached to the C atom of the C=C bond then only it would lead to giving only ketone on ozonolysis.
13. Which are the correct IUPAC names of the following compound?
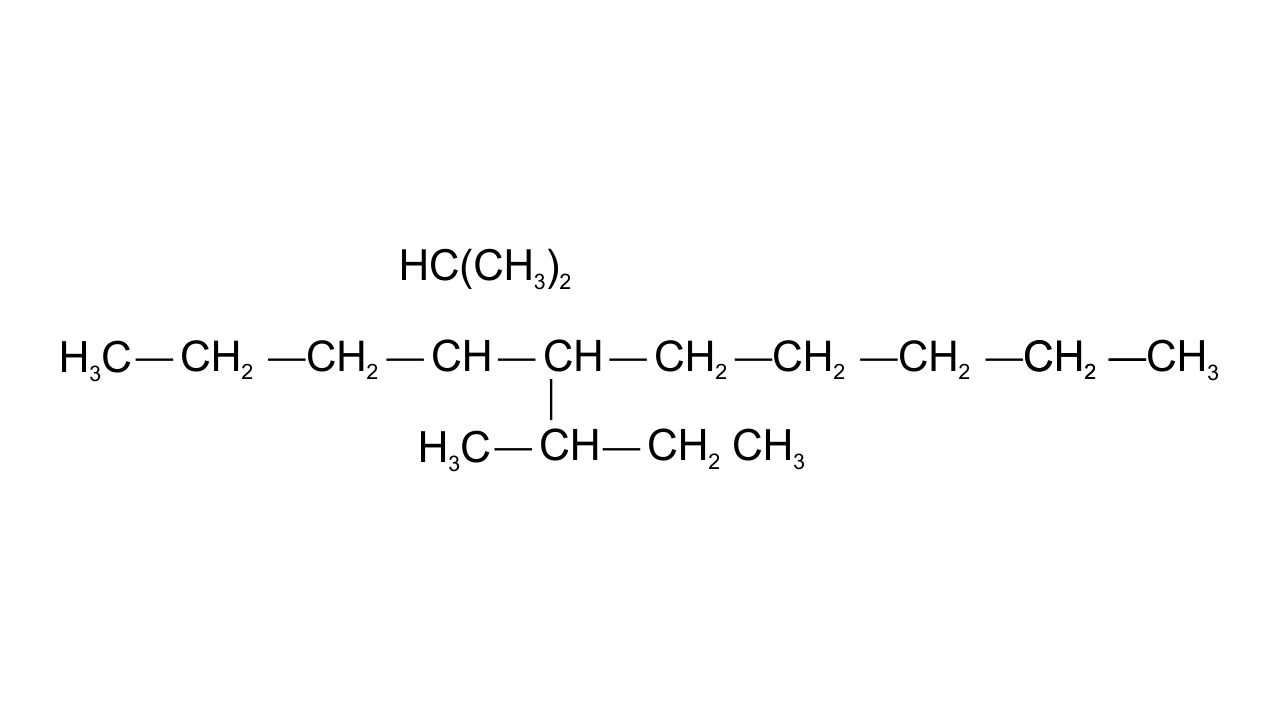
(i) 5– Butyl – 4– isopropyldecane
(ii) 5– Ethyl – 4– propyldecane
(iii) 5– sec-Butyl – 4– iso-propyldecane
(iv) 4–(1-methylethyl)– 5 – (1-methylpropyl)-decane
Ans:(iii) & (iv)
The longest carbon chain is of 10 carbon atom
14. Which are the correct IUPAC names of the following compound?

Ans: (i) & (iv)
The longest carbon chain is of 10 carbon atom
15. For an electrophilic substitution reaction, the presence of a halogen atom in the benzene ring _______.
(i) deactivates the ring by inductive effect
(ii) deactivates the ring by resonance
(iii) increases the charge density at ortho and para position relative to meta position by resonance
(iv) directs the incoming electrophile to meta position by increasing the charge density relative to ortho and para position.
Ans: (i) & (iii)
The halogens atom weakly deactivate the ring due to the -I effect and directs the incoming group to the ortho and para position due to the +R effect.
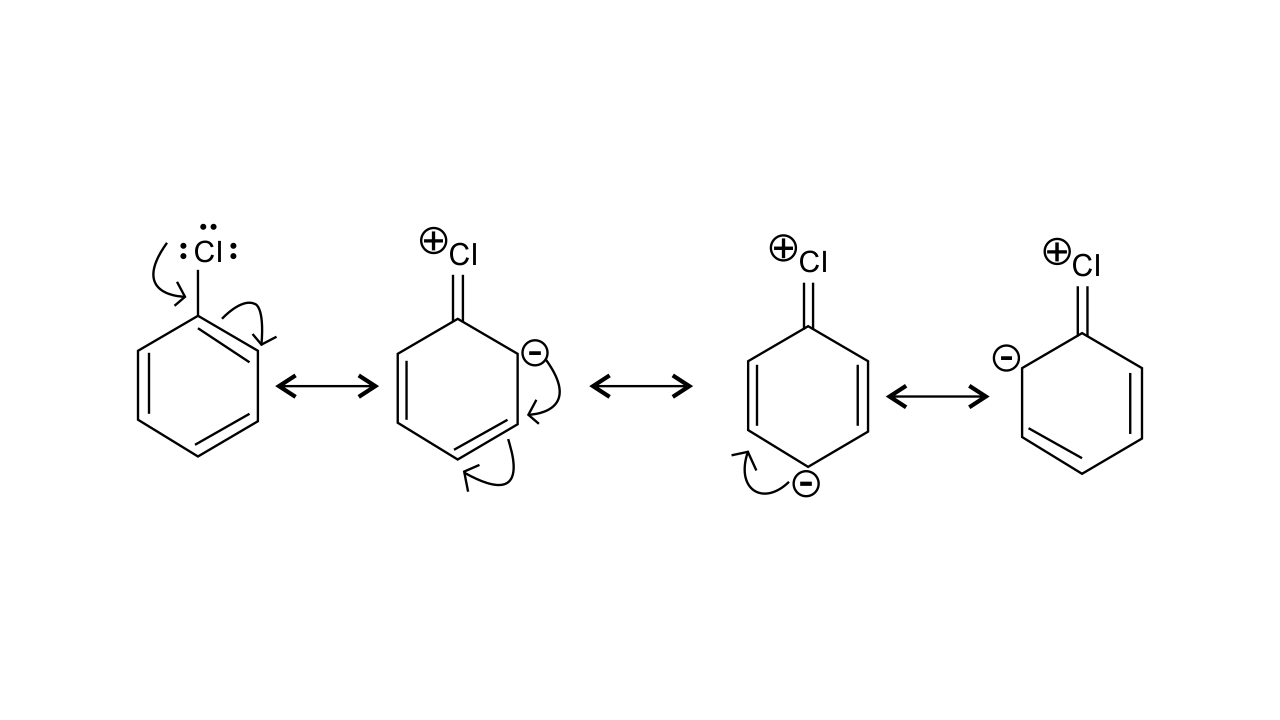
16. In an electrophilic substitution reaction of nitrobenzene, the presence of nitro group ________.
(i) deactivates the ring by inductive effect.
(ii) activates the ring by inductive effect.
(iii) decreases the charge density at ortho and para position of the ring relative to meta position by resonance.
(iv) increases the charge density at meta position relative to the ortho and para positions of the ring by resonance
Ans: (i) & (iii)
The nitro group strongly deactivate the ring due to the -R effect and -I effect
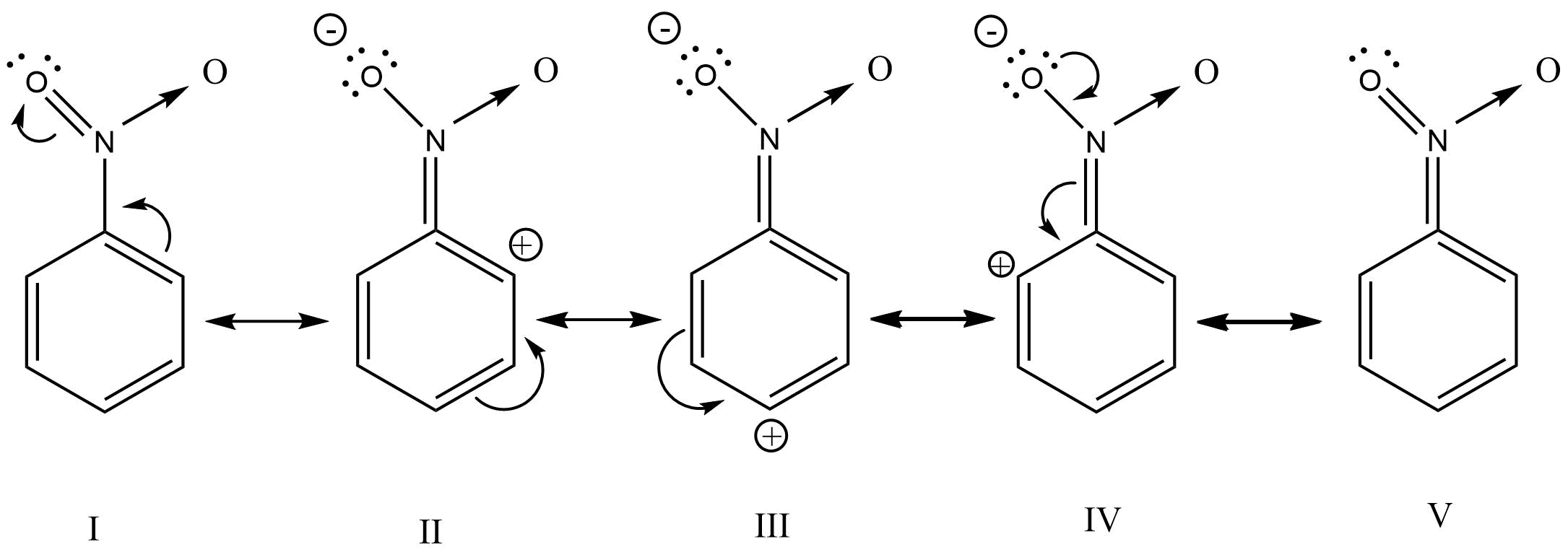
17. Which of the following are correct?
(i) CH3—O—CH2⊕ is more stable than CH3—CH2⊕
(ii) (CH3)2CH⊕ is less stable than CH3—CH2—CH2⊕
(iii) CH2=CH—CH2⊕ is more stable than CH3—CH2—CH2⊕
(iv) CH2=CH⊕is more stable than CH3—CH2⊕
Ans: (i) & (iii)
The +I effect of the -OCH3 group stabilizes the carbocation.
The two resonating structure of CH2=CH-CH2⊕↔⊕CH2-CH=CH2 makes it more stable
18. Four structures are given in options (i) to (iv). Examine them and select the aromatic structures.

Ans: (i) & (iii)
The structures that follow the (4n+2) Huckle rule of aromaticity is considered to be aromatic
19. The molecules having dipole moment are __________.
(i) 2,2-Dimethylpropane
(ii) trans-Pent-2-ene
(iii) cis-Hex-3-ene
(iv) 2, 2, 3, 3 – Tetramethylbutane.
Ans: (ii) & (iii)

Short Answer Type
20. Why do alkenes prefer to undergo electrophilic addition reactions while arenes prefer electrophilic substitution reactions? Explain.
Ans: If arenes would undergo electrophilic addition reaction then they would lose their aromaticity which would lead to comparatively less stability. However, alkenes undergo electrophilic addition reaction via carbocation intermediate.
21. Alkynes on reduction with sodium in liquid ammonia form trans alkenes. Will the butene thus formed on reduction of 2-butyne show the geometrical isomerism?
Ans: Yes, it will execute geometrical isomerism as the product formed can be either cis-2-butene or trans-2-butene.

22. Rotation around carbon-carbon single bond of ethane is not completely free. Justify the statement.
Ans: Therotation around C-C single bond is possible but it is not completely free due to the torsional strain which is about 1-20 KJ mol-1.
23. Draw Newman and Sawhorse projections for the eclipsed and staggered conformations of ethane. Which of these conformations is more stable and why?
Ans:

The staggered conformation is more stable because of comparatively less torsional strain.
24. The intermediate carbocation formed in the reactions of HI, HBr and HCl with propene is the same and the bond energy of HCl, HBr and HI is 430.5 kJ mol–1, 363.7 kJ mol–1 and 296.8 kJ mol–1 respectively. What will be the order of reactivity of these halogen acids?
Ans: The rate-determining step involved in the reaction is
CH3-CH=CH2 + HX → CH3-CH+-CH3 + X–
So the rate-determining step depends on the bond energy of HX i.e. higher the bond energy lesser would be reactivity.
Thus the order of reactivity of these halogen acids is
HI>HBr>HCl
25. What will be the product obtained as a result of the following reaction and why?
Ans: Thebenzene here would undergo Friedel-Crafts alkylation reaction where carbocation is formed as an intermediate, thus secondary carbocation is formed as an intermediate due to its greater stability than that of the primary carbocation.
The final product obtained is
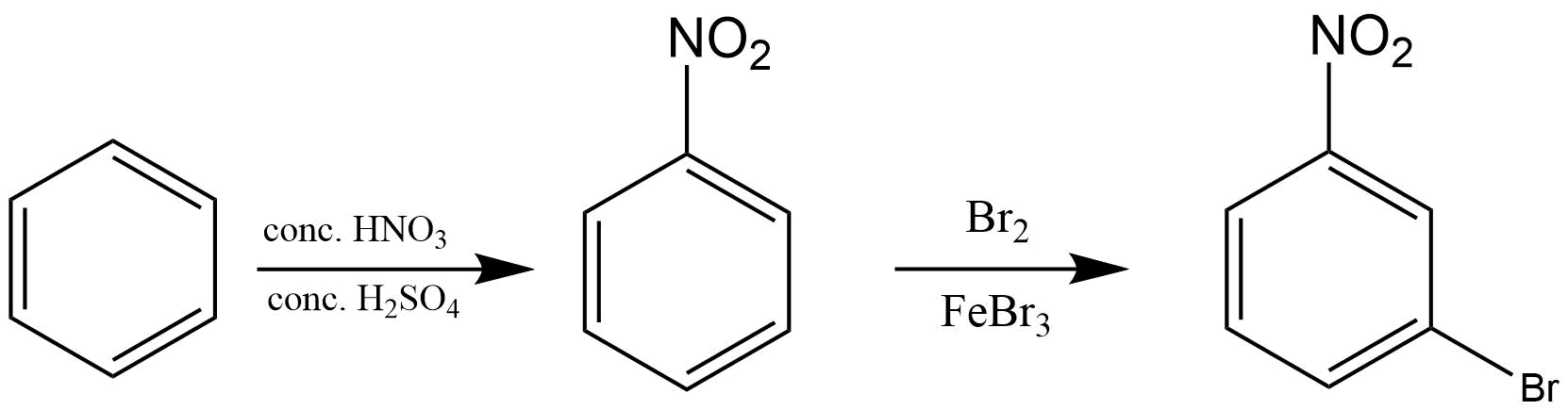
26. How will you convert benzene into
(i) p – nitrobromobenzene
(ii) m – nitrobromobenzene
Ans:
(i)
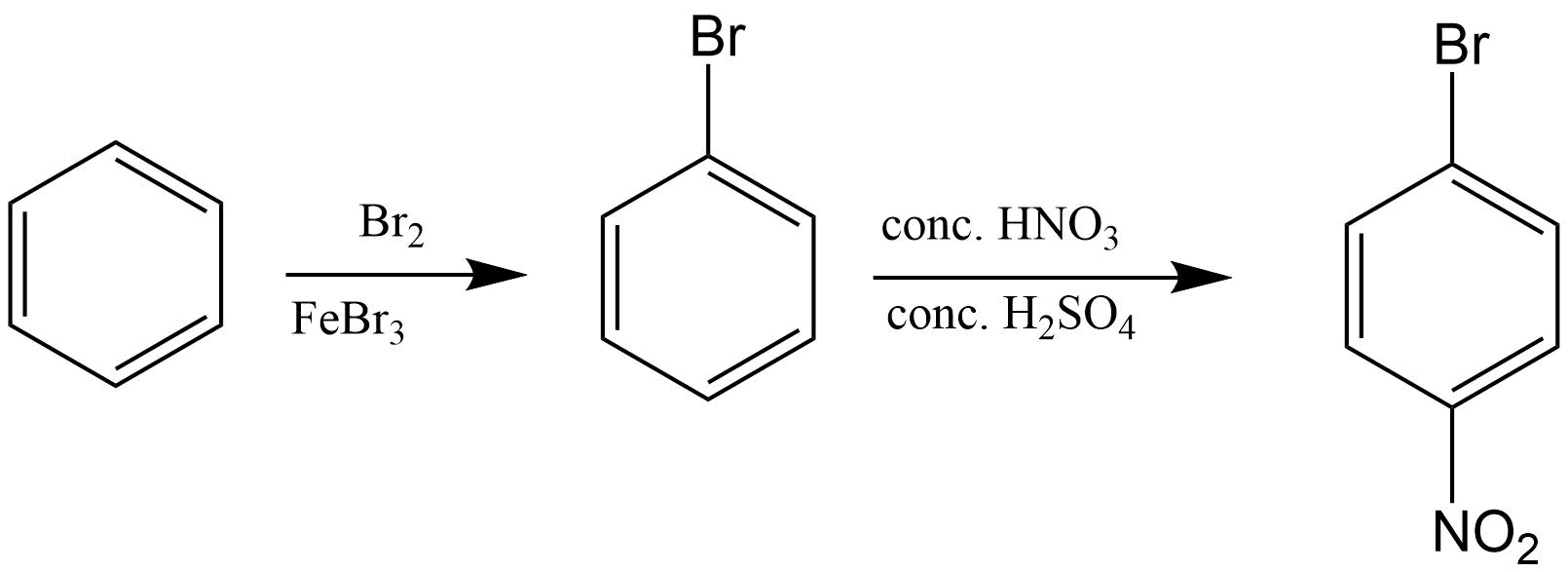
(ii)
27. Arrange the following set of compounds in the order of their decreasing relative reactivity with an electrophile.Give reason

Ans: The electron donating group increases the reactivity in electrophilic substitution reaction whereas the electron withdrawing group decreases the reactivity in electrophilic substitution.
Thus, the order of reactivity
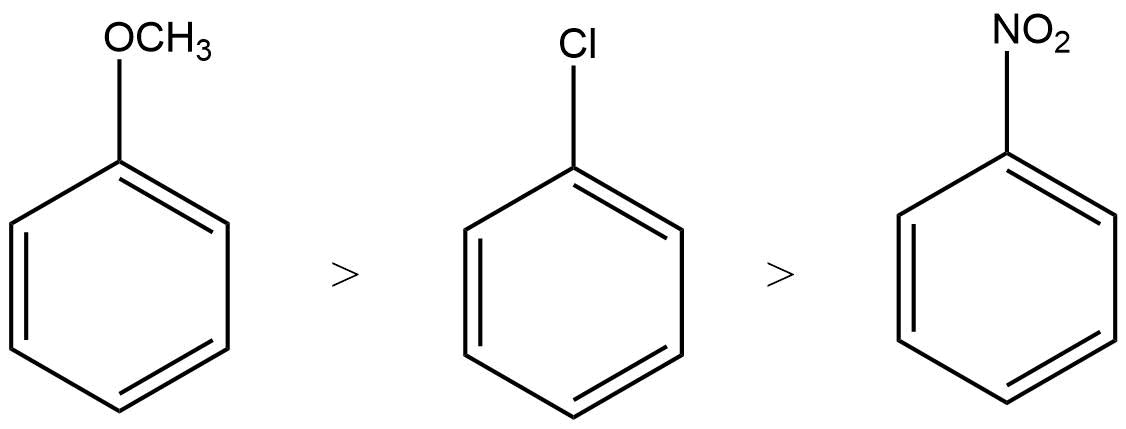
28. Despite their – I effect, halogens are o- and p-directing in haloarenes. Explain.
Ans: Due to the +R effect halogens are ortho and para directing groups.

29. Why does presence of a nitro group make the benzene ring less reactive in comparison to the unsubstituted benzene ring. Explain.
Ans: The nitro group strongly deactivate the benzene towards the electrophilic substitution reaction due to the -R and -I effect
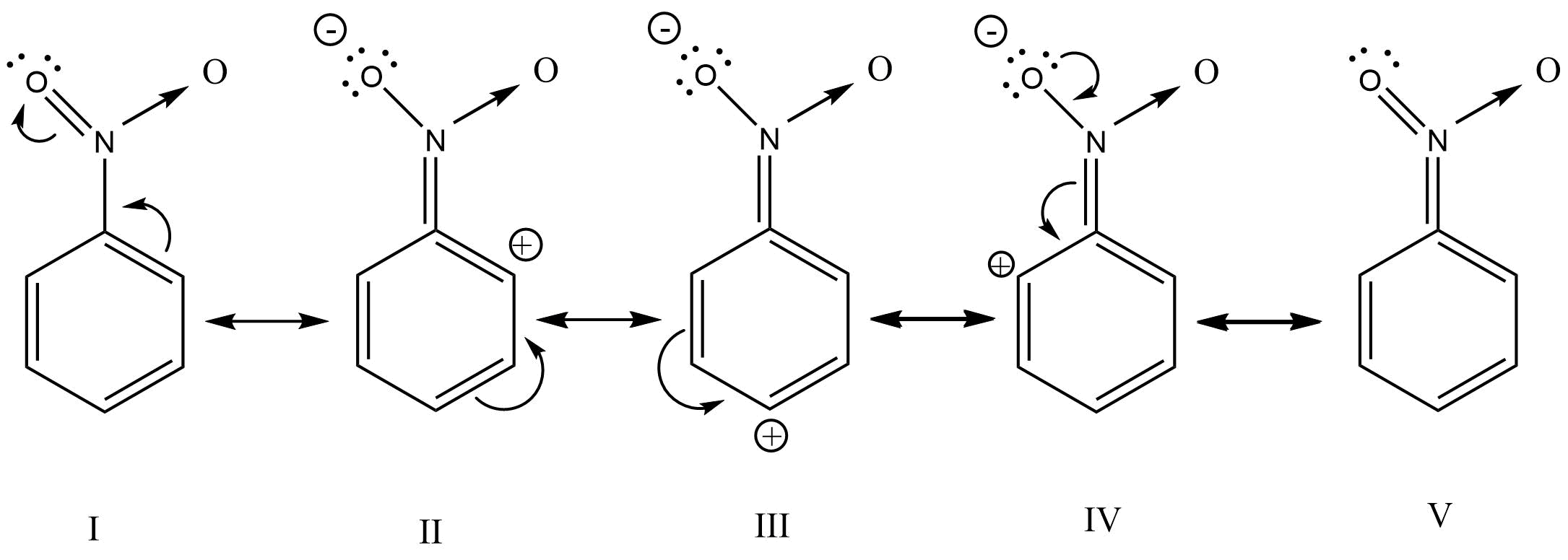
30. Suggest a route for the preparation of nitrobenzene starting from acetylene?
Ans:

31. Predict the major product (s) of the following reactions and explain their formation.
Ans: In presence of (Ph-CO-O)2it lead to CH3-CH2-CH2Br as the reaction undergo by free radical mechanism
However in absence of (Ph-CO-O)2 the reaction undergo by carbocation intermediate thus lead to give CH3-CHBr-CH3
32. Nucleophiles and electrophiles are reaction intermediates having electron rich and electron deficient centres respectively. Hence, they tend to attack electron deficient and electron rich centres respectively. Classify the following species as electrophiles and nucleophiles.
(i) H3CO–
(ii)
(iii)
(iv)
(v) (H3C)3C+
(vi) Br–
(vii) H3COH
(viii) R-NH-R
Ans:
| Electrophiles | Nucleophiles |
|---|---|
| (iii),(iv),(v) | (i),(ii),(vi),(vii),(viii) |
33. The relative reactivity of 1°, 2°, 3° hydrogen’s towards chlorination is 1 : 3.8 : 5. Calculate the percentages of all monochlorinated products obtained from 2-methylbutane.
Ans: The structure of 2-methylbutane is
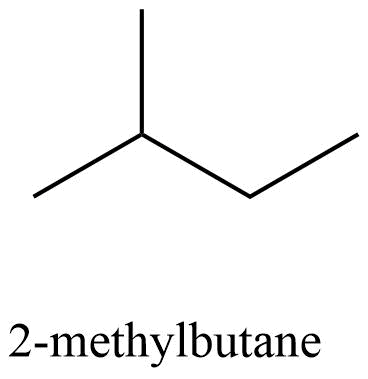
| Total number | Total amount of monochlorinated
Product |
% of monochlorinated product | |
|---|---|---|---|
| 10 | 9 | 9 | 41.7 |
| 20 | 2 | 7.6 | 35.2 |
| 30 | 1 | 5 | 23.1 |
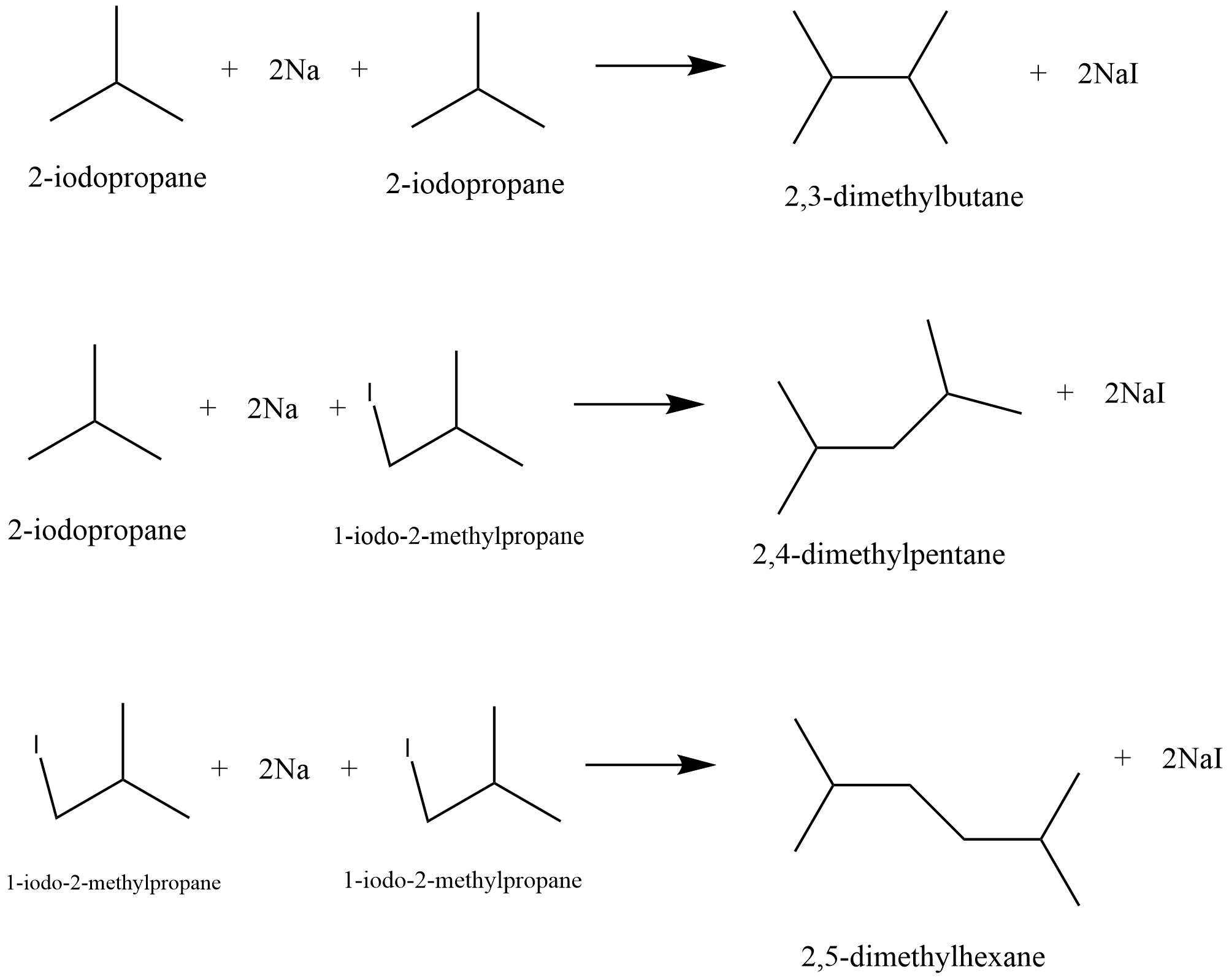
34. Write the structures and names of products obtained in the reactions of sodium with a mixture of 1-iodo-2-methylpropane and 2-iodopropane.
Ans: It is a Wurtzreaction where alkane is formed.
35. Write hydrocarbon radicals that can be formed as intermediates during monochlorination of 2-methylpropane? Which of them is more stable? Give reasons
Ans:The 2-methylpropane would lead to two types of radicals.
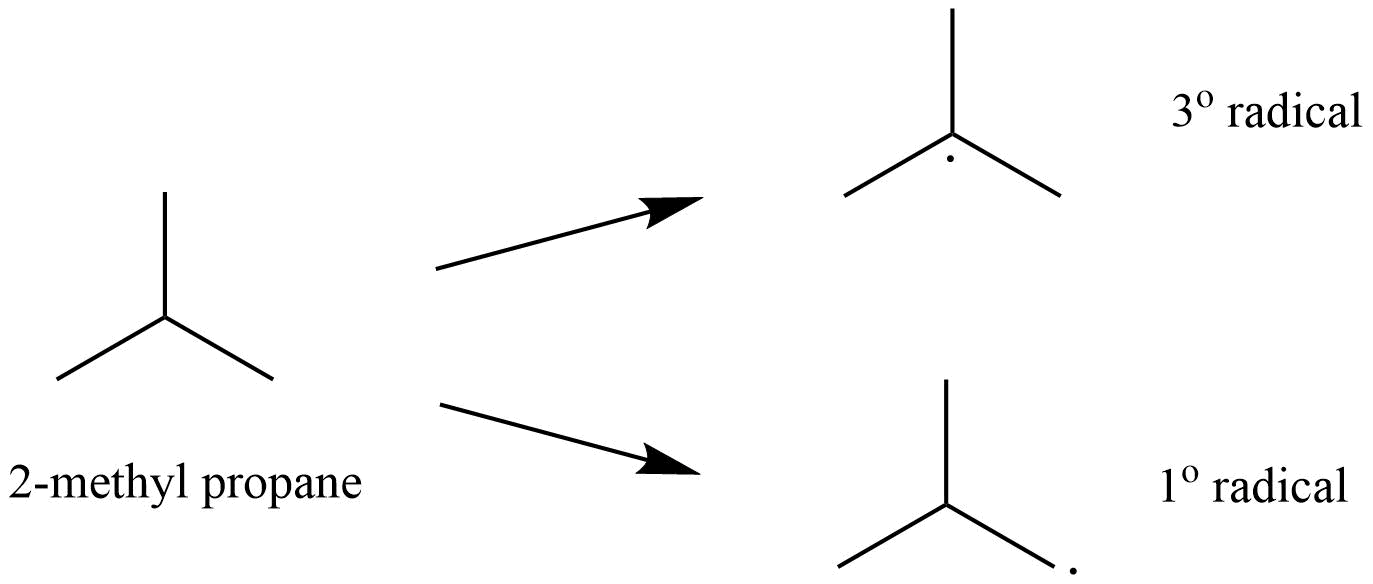
Among the two 3o radical is most stable as it contains 9 ∝ -hydrogen whereas 1o radical contains only 1 ∝ -hydrogen.
36. An alkane C8H18 is obtained as the only product on subjecting a primary alkyl halide to Wurtz reaction. On monobromination this alkane yields a single isomer of a tertiary bromide. Write the structure of alkane and the tertiary bromide.
Ans: The reaction is

The alkane is
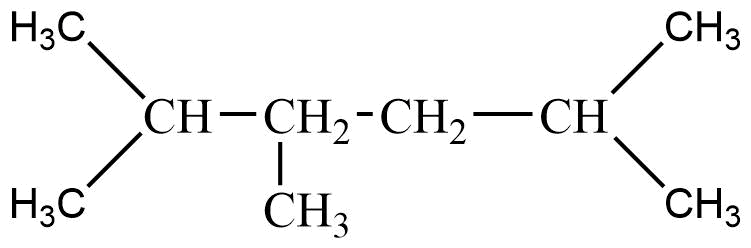
The tertiary bromide is

37. The ring systems having following characteristics are aromatic.
(i) Planar ring containing conjugated π bonds.
(ii) Complete delocalisation of the π−electrons in ring system i.e. each atom in the ring hasunhybridised p-orbital, and
(iii) Presence of (4n+2) π−electrons in the ring where n is an integer (n = 0, 1, 2,………..)
Huckelrule
Huckelrule.
Using this information classify the following compounds as aromatic/nonaromatic.

Ans:
| Aromatic | Non-aromatic |
|---|---|
| A,C,E,F | B,D,G |
38. Which of the following compounds are aromatic according to Huckel’s rule?
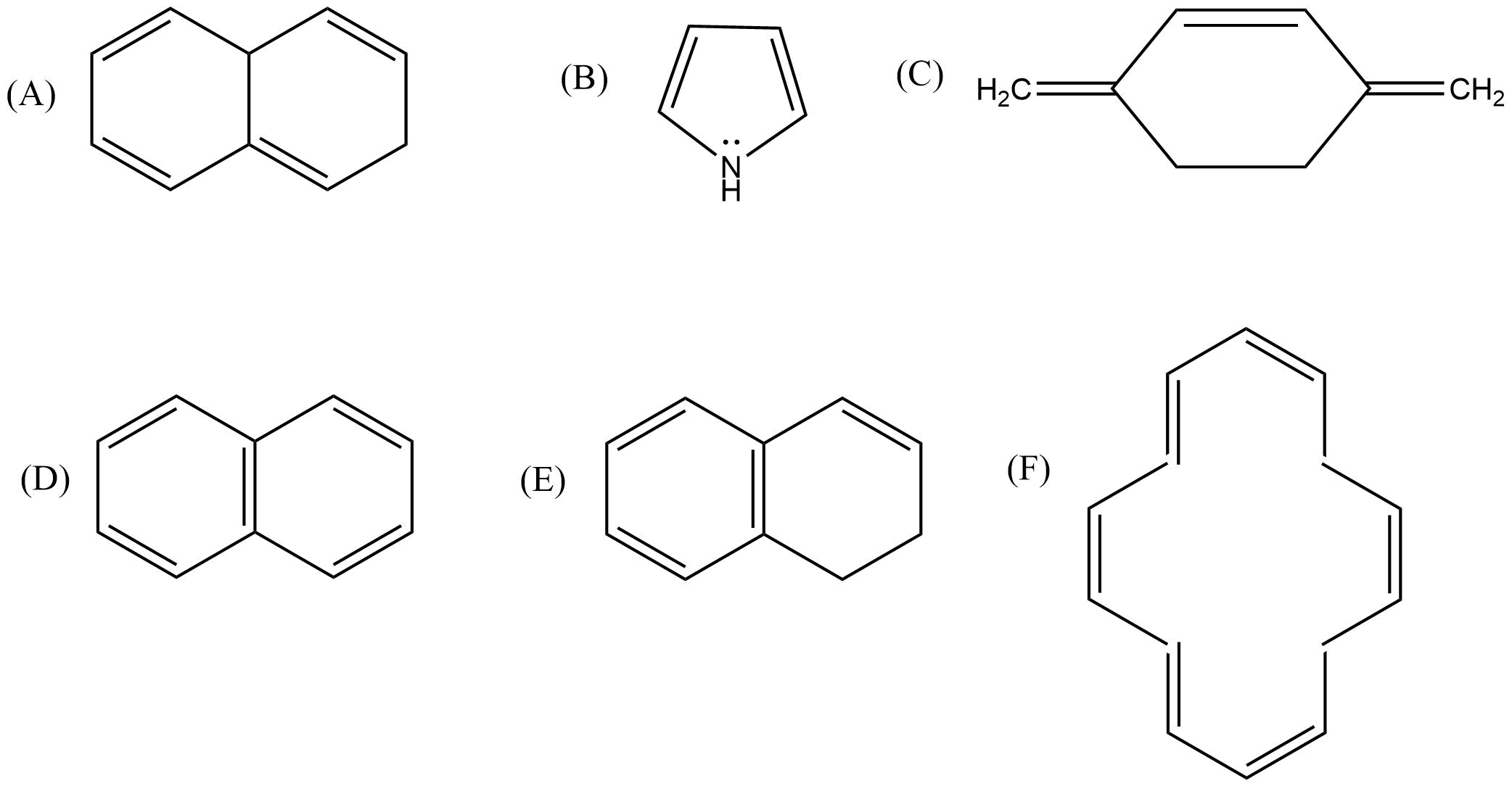
Ans: As per the Huckel (4n+2) rule following distinction can be made:-
| Aromatic | Non aromatic |
|---|---|
| B,D,F | A,C,E |
39. Suggest a route to prepare ethyl hydrogensulphate (CH3–CH2–OSO2—OH) starting from ethanol (C2H5OH).
Ans:

Matching Type
40. Match the reagent from Column I which on reaction with
CH3 – CH=CH2 gives some product given in Column II as per the codes given below:
| Column I | Column II |
|---|---|
| (i) O3/Zn + H2O | (a) Acetic acid and CO2 |
| (ii) KMnO4/H+ | (b) Propan-1-ol |
| (iii) KMnO4/OH– | (c) Propan-2-ol |
| (iv) H2O/H+ | (d) Acetaldehyde and formaldehyde |
| (v) B2H6/NaOH and H2O2 | (e) Propan-1,2-diol |
Ans:
| (i) O3/Zn + H2O | (d) Acetaldehyde and formaldehyde |
|---|---|
| (ii) KMnO4/H+ | (a)Acetic acid and CO2 |
| (iii) KMnO4/OH– | (e) Propan-1,2-diol |
| (iv) H2O/H+ | (c) Propan-2-ol |
| (v) B2H6/NaOH and H2O2 | (b) Propan-1-ol |
41. Match the hydrocarbons in Column I with the boiling points given in Column II.
| Column I | Column II |
|---|---|
| (i) n-pentane | (a) 282.5 K |
| (ii) iso-pentane | (b) 309 K |
| (iii) neo-pentane | (c) 301 K |
Ans:
| (i) n-pentane | (b) 309 K |
|---|---|
| (ii) iso-pentane | (c) 301 K |
| (iii) neo-pentane | (a) 282.5 K |
42. Match the following reactants in Column I with the corresponding reaction products in Column II.
| Column I | Column II |
|---|---|
| (i) Benzene + Cl2
− → − − AlC l 3 →AlCl3 |
(a) Benzoic acid |
| (ii)Benzene + CH3Cl
− → − − AlC l 3 →AlCl3 |
(b) Methyl phenyl ketone |
| (iii)Benzene + CH3COCl
− → − − AlC l 3 →AlCl3 |
(c) Toluene |
| (iv)Toluene
− → − − − − − − − − KMn o 4 /NaOH →KMno4/NaOH |
(d) Chlorobenzene |
| (e) Benzene hexachloride |
Ans:
| (i) Benzene + Cl2
− → − − AlC l 3 →AlCl3 |
(d) Chlorobenzene |
|---|---|
| (ii)Benzene + CH3Cl
− → − − AlC l 3 →AlCl3 |
(c) Toluene |
| (iii)Benzene + CH3COCl
− → − − AlC l 3 →AlCl3 |
(b) Methyl phenyl ketone |
| (iv)Toluene
− → − − − − − − − − KMn o 4 /NaOH →KMno4/NaOH |
(a) Benzoic acid |
43. Match the reactions given in Column I with the reaction types in Column II.
| Column I | Column II |
|---|---|
| (i) CH2=CH2 + H2O
− → − H + →H+ CH3CH2OH |
(a) Hydrogenation |
| (ii) CH2 ≡ CH2 + H2
− → Pd →Pd CH3CH3 |
(b) Halogenation |
| (iii) CH2 ≡ CH2 + Cl2 → ClCH2CH2Cl | (c) Polymerisation |
| (iv) 3CH ≡ CH
− → − − − − tube heat Cu →tube heatCu C6H6 |
(d) Hydration |
| (e) Condensation |
Ans:
| (i) CH2=CH2 + H2O
− → − H + →H+ CH3CH2OH |
(d) Hydration |
|---|---|
| (ii) CH2=CH2 + H2
− → Pd →Pd CH3CH3 |
(a) Hydrogenation |
| (iii) CH2=CH2 + Cl2→ClCH2CH2Cl | (b) Halogenation |
| (iv) 3CH ≡ CH
− → − − − − tube heat Cu →tube heatCu C6H6 |
(c) Polymerisation |
Assertion and Reason Type
In the following questions a statement of assertion (A) followed by a statement of reason (R) is given. Choose the correct option out of the choices given below each question.
44. Assertion (A): The compound cyclooctane has the following structural formula:

It is cyclic and has conjugated 8π-electron system but it is not an aromatic compound.
Reason (R): (4n + 2) π electrons rule does not hold good and ring is not planar.
(i) Both A and R are correct and R is the correct explanation of A.
(ii) Both A and R are correct but R is not the correct explanation of A.
(iii) Both A and R are not correct.
(iv) A is not correct but R is correct.
Ans: (i)
Because cyclooctatetraene is tubbed shaped.
45. Assertion (A): Toluene on Friedal Crafts methylation gives o– and p–xylene.
Reason (R): CH3-group bonded to benzene ring increases electron density at o– and p– position.
(i) Both A and R are correct and R is the correct explanation of A.
(ii) Both A and R are correct but R is not the correct explanation of A.
(iii) Both A and R are not correct.
(iv) A is not correct but R is correct.
Ans:
(i) The -CH3 group is an activating group and it directs the upcoming substituent to the ortho and para position.
46. Assertion (A): Nitration of benzene with nitric acid requires the use of concentrated sulphuric acid.
Reason (R): The mixture of concentrated sulphuric acid and concentrated nitric acid produces the electrophile, NO2 +.
(i) Both A and R are correct and R is the correct explanation of A.
(ii) Both A and R are correct but R is not the correct explanation of A.
(iii) Both A and R are not correct.
(iv) A is not correct but R is correct.
Ans: (i)
The sulphuric acid helps in furnishing NO2+(electrophile).

47. Assertion (A): Among isomeric pentanes, 2, 2-dimethylpentane has highest boiling point.
Reason (R): Branching does not affect the boiling point.
(i) Both A and R are correct and R is the correct explanation of A.
(ii) Both A and R are correct but R is not the correct explanation of A.
(iii) Both A and R are not correct.
(iv) A is not correct but R is correct.
Ans:(iii)
With the branching the boiling decreases due to the decrease in surface area which leads to a decrease its van der Waals force.
Long Answer Type
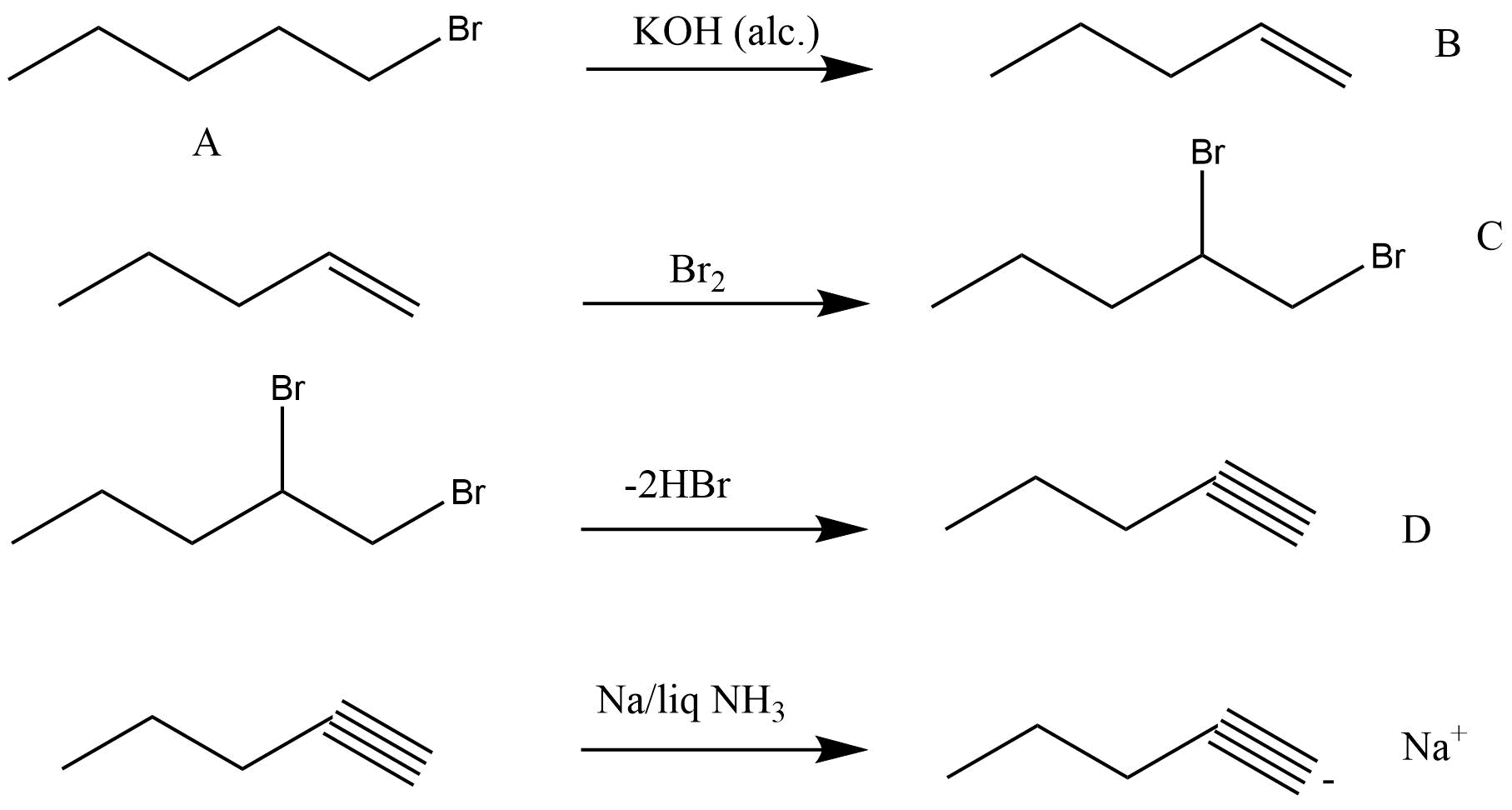
48. An alkyl halide C5H11Br (A) reacts with ethanolic KOH to give an alkene ‘B’, which reacts with Br2 to give a compound ‘C’, which on dehydrobromination gives an alkyne ‘D’. On treatment with sodium metal in liquid ammonia one mole of ‘D’ gives one mole of the sodium salt of ‘D’ and half a mole of hydrogen gas. Complete hydrogenation of ‘D’ yields a straight chain alkane. Identify A,B, C and D. Give the reactions invovled.
Ans:
49. 896 mL vapour of a hydrocarbon ‘A’ having carbon 87.80% and hydrogen 12.19% weighs 3.28g at STP. Hydrogenation of ‘A’ gives 2-methylpentane. Also ‘A’ on hydration in the presence of H2SO4 and HgSO4 gives a ketone ‘B’ having molecular formula C6H12O. The ketone ‘B’ gives a positive iodoform test. Find the structure of ‘A’ and give the reactions involved.
Ans: The molar mass of the compound =
3.28×22400
896
3.28×22400896
= 831gmol-1
| Element | Percentage | At.mass | Relative ratio | Simplest ratio |
|---|---|---|---|---|
| C | 87.8% | 12 | 7.31 | 3 |
| H | 12.19% | 1 | 12.19 | 5 |
Thus the empirical formula of the compound is C3H5
Molecular formula =n x empirical formula = (831/41) x C3H5 = C6H10

50. An unsaturated hydrocarbon ‘A’ adds two molecules of H2 and on reductive ozonolysis gives butane-1,4-dial, ethanal and propanone. Give the structure of ‘A’, write its IUPAC name and explain the reactions involved.
Ans: The structure of A is
The reaction involved are as follows:

51. In the presence of peroxide addition of HBr to propene takes place according to anti Markovnikov’s rule but peroxide effect is not seen in the case of HCl and HI. Explain.
Ans: The bond energy of HCl is higher than that of HBr thus it is not cleaved by free radical mechanism to exhibit peroxide effect. However in case of HI the bond energy is so low that the iodine radical forms readily and after formation it combines to form an iodine molecule.
NCERT Exemplar For Class 11 Science
The NCERT exemplars are an effective study material for scoring higher marks in the examination paper. Students must practise these additional questions for their own benefits, as these are curated by the best subject-matter experts. Students can easily access the ncert exemplar for class 11 science by visiting our website SimplyAcad and solve all the questions listed to secure maximum marks.
Here are some other NCERT exemplar for class 11 chemistry:
| NCERT exemplar for class 11 chemistry Chapter 1 | NCERT exemplar for class 11 chemistry Chapter 6 |
|---|---|
| NCERT exemplar for class 11 chemistry Chapter 2 | NCERT exemplar for class 11 chemistry Chapter 7 |
| NCERT exemplar for class 11 chemistry Chapter 3 | NCERT exemplar for class 11 chemistry Chapter 9 |
| NCERT exemplar for class 11 chemistry Chapter 4 | NCERT exemplar for class 11 chemistry Chapter 10 |
| NCERT exemplar for class 11 chemistry Chapter 5 | NCERT exemplar for class 11 chemistry Chapter 11 |
latest video
news via inbox
Nulla turp dis cursus. Integer liberos euismod pretium faucibua