NCERT Exemplar Class 12 Chemistry Chapter 11 Alcohols Phenols And Ethers
NCERT Exemplar for Class 12 Chemistry Chapter 11
SimplyAcad has provided the NCERT Exemplar for Class 12 Chemistry below to help students learn all the chapters in a detailed manner. The exemplar will allow students to gain deep insights of all the sections and prepares you for the upcoming examination. Chemistry is an interesting subject which requires attention to minor details, hence, completing exemplars will be an effective way to increase your marks and confidence. The given exemplar contains MCQs with two different types, and Short Answer Type Questions, and contains a total of 56 questions. Students can access the NCERT exemplar for class 12 chemistry, Chapter 11 Alcohols, Phenols and Ethers by scrolling below. Along with this, there are several NCERT exemplar for class 12 science of all the chapters provided on this platform.
Access the NCERT Exemplar Class 12 Chemistry Chapter 11 Alcohols, Phenols and Ethers
I. Multiple Choice Questions (Type-I)
1. Monochlorination of toluene in sunlight followed by hydrolysis with aq. NaOH yields.
(i) o-Cresol
(ii) m-Cresol
(iii) 2, 4-Dihydroxytoluene
(iv) Benzyl alcohol
Solution:
Option (iv) is the answer.
2. How many alcohols with molecular formula C4H10O are chiral?
(i) 1
(ii) 2
(iii) 3
(iv) 4
Solution:
Option (i) is the answer.
3. What is the correct order of reactivity of alcohols in the following reaction?
R—OH + HCl →(ZnCl2) R—Cl + H2O
(i) 1° > 2° > 3°
(ii) 1° < 2° > 3°
(iii) 3° > 2° > 1°
(iv) 3° > 1° > 2°
Solution:
Option (iii) is the answer.
4. CH3CH2OH can be converted into CH3CHO by ______________.
(i) catalytic hydrogenation
(ii) treatment with LiAlH4
(iii) treatment with pyridinium chlorochromate
(iv) treatment with KMnO4
Solution:
Option (iii) is the answer.
5. The process of converting alkyl halides into alcohols involves_____________.
(i) addition reaction
(ii) substitution reaction
(iii) dehydrohalogenation reaction
(iv) rearrangement reaction
Solution:
Option (ii) is the answer.
6. Which of the following compounds is aromatic alcohol?

(i) A, B, C, D
(ii) A, D
(iii) B, C
(iv) A
Solution:
Option (iii) is the answer.
7. Give IUPAC name of the compound given below.

(i) 2-Chloro-5-hydroxyhexane
(ii) 2-Hydroxy-5-chlorohexane
(iii) 5-Chlorohexan-2-ol
(iv) 2-Chlorohexan-5-ol
Solution:
Option (iii) is the answer.
8. IUPAC name of m-cresol is ___________.
(i) 3-methylphenol
(ii) 3-chlorophenol
(iii) 3-methoxyphenol
(iv) benzene-1,3-diol
Solution:
Option (i) is the answer.
9. IUPAC name of the compound is ______________.
(i) 1-methoxy-1-methylethane
(ii) 2-methoxy-2-methylethane
(iii) 2-methoxypropane
(iv) isopropylmethyl ether
Solution:
Option (iii) is the answer.
10. Which of the following species can act as the strongest base?

Solution:
Option (ii) is the answer.
11. Which of the following compounds will react with sodium hydroxide solution in water?
(i) C6H5OH
(ii) C6H5CH2OH
(iii) (CH3)3 COH
(iv) C2H5OH
Solution:
Option (i) is the answer
12. Phenol is less acidic than ______________.
(i) ethanol
(ii) o-nitrophenol
(iii) o-methyl phenol
(iv) o-methoxy phenol
Solution:
Option (ii) is the answer.
13. Which of the following is most acidic?
(i) Benzyl alcohol
(ii) Cyclohexanol
(iii) Phenol
(iv) m-Chlorophenol
Solution:
Option (iv) is the answer.
14. Mark the correct order of decreasing acid strength of the following compounds.

(i) e > d > b > a > c
(ii) b > d > a > c > e
(iii) d > e > c > b > a
(iv) e > d > c > b > a
Solution:
Option (ii) is the answer.
15. Mark the correct increasing order of reactivity of the following compounds with HBr/HCl.

(i) a < b < c
(ii) b < a < c
(iii) b < c < a
(iv) c < b < a
Solution:
Option (iii) is the answer.
16. Arrange the following compounds in increasing order of boiling point. Propan-1-ol, butan-1-ol, butan-2-ol, pentan-1-ol
(i) Propan-1-ol, butan-2-ol, butan-1-ol, pentan-1-ol
(ii) Propan-1-ol, butan-1-ol, butan-2-ol, pentan-1-ol
(iii) Pentan-1-ol, butan-2-ol, butan-1-ol, propan-1-ol
(iv) Pentan-1-ol, butan-1-ol, butan-2-ol, propan-1-ol
Solution:
Option (i) is the answer
II. Multiple Choice Questions (Type-II)
Note: In the following questions, two or more options may be correct.
17. Which of the following are used to convert RCHO into RCH2OH?
(i) H2/Pd
(ii) LiAlH4
(iii) NaBH4
(iv) Reaction with RMgX followed by hydrolysis
Solution:
Option (i), (ii) and (iii) are the answers.
18. Which of the following reactions will yield phenol?

Solution:
Option (A), (B) and (C)
19. Which of the following reagents can be used to oxidise primary alcohols to aldehydes?
(i) CrO3
in an anhydrous medium.
(ii) KMnO4 in acidic medium.
(iii) Pyridinium chlorochromate.
(iv) Heat in the presence of Cu at 573K
Solution:
Option (i), (iii) and (iv) are the answers.
20. Phenol can be distinguished from ethanol by the reactions with _________.
(i) Br2/water
(ii) Na
(iii) Neutral FeCl3
(iv) All the above
Solution:
Option (i) and (iii) are the answers.
21. Which of the following are benzylic alcohols?
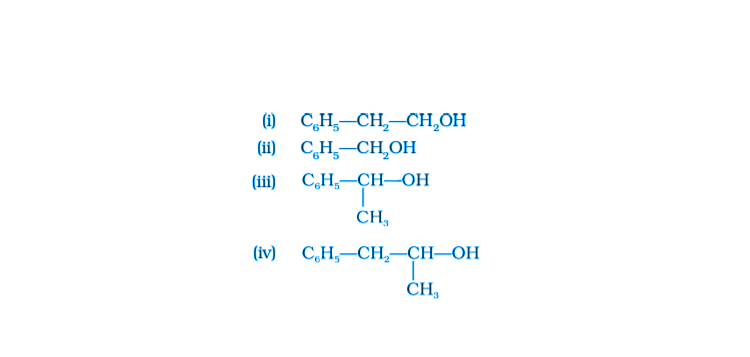
Solution:
Option (ii) and (iii) are the answers.
III. Short Answer Type
22. What are the structure and IUPAC name of glycerol?
Solution:
Structure of Glycerol

IUPAC name: Propane-1,2,3-triol
23. Write the IUPAC name of the following compounds.

Solution:
(A) IUPAC name of the compound is 3-Ethyl-5-methyl hexane-2,4-diol.
(B) IUPAC name of the compound is1-Methoxy-3-nitrocyclohexane.
24. Write the IUPAC name of the compound given below.

Solution:
The IUPAC name of the compound is 3-Methylpent-2-ene-1,2-diol.
25. Name the factors responsible for the solubility of alcohols in water.
Solution:
The factors responsible for the solubility of alcohols in water are:
I) Hydrogen bonds
II) Size of the alkyl or aryl groups
III) The molecular mass of the Alcohols.
26. What is denatured alcohol?
Solution:
Alcohols that are used for drinking are made unfit for human consumption by mixing alcohol with some copper sulfate and pyridine, which gives the colour and a foul smell to the liquid, respectively. This is called denatured alcohol.
27. Suggest a reagent for the following conversion.

Solution:
The chemical reaction above shows the oxidation of secondary alcohol into a ketone. This can be easily achieved by using oxidizing agents like chromic anhydride (CrO3), Pyridinium chlorochromate (PCC), etc.
28. Out of 2-chloroethanol and ethanol, which is more acidic and why?
Solution:
2-chloroethanol is more acidic because of the presence of chlorine which is an electron-withdrawing group. This results in a negative inductive effect, and thus, the electron density in the –O-H bond decreases. It stabilizes the alkoxide ion, and therefore, 2-chloroethanol can easily release a proton.
29. Suggest a reagent for the conversion of ethanol to ethanal.
Solution:
PCC or Pyridinium chlorochromate can be used as reagents. The above conversion shows the oxidation of primary alcohol to an aldehyde.
30. Suggest a reagent for the conversion of ethanol to ethanoic acid.
Solution:
Acidified KMnO4 can be used as a reagent for the above conversion. The above conversion shows the oxidation of primary alcohol to a carboxylic acid.
31. Out of o-nitrophenol and p-nitrophenol, which is more volatile? Explain.
Solution:
Due to the presence of intramolecular hydrogen bonding between the NO2 and OH group, the o-nitrophenol is more volatile.
32. Out of o-nitrophenol and o-cresol, which is more acidic?
Solution:
There is an electron-withdrawing group NO2 that is present in the ortho position in ortho-nitrophenol, which enhances the acidic strength and makes it more acidic. In o-cresol, there is an electron-releasing group that decreases acidic strength.
33. When phenol is treated with bromine water, a white precipitate is obtained. Give the structure and the name of the compound formed.
Solution:
The name of the compound formed in this reaction is 2,4,6-tribromophenol. The structure of the compound formed is:

34. Arrange the following compounds in increasing order of acidity and give a suitable explanation. Phenol, o-nitrophenol, o-cresol
Solution:
Increasing order of the acidity of the given compounds: o-cresol < Phenol < o-nitrophenol.
Due to the presence of the electron-withdrawing group, NO2 o-nitrophenol becomes more acidic. The remaining has an electron-releasing group which decreases the acidic strength.
35. Alcohols react with active metals e.g. Na, K etc., to give corresponding alkoxides. Write down the decreasing order of reactivity of sodium metal towards primary, secondary and tertiary alcohols
Solution:
The decreasing order of reactivity of sodium metal towards alcohols: Primary alcohols > secondary alcohols > tertiary alcohols
The reactivity of sodium metal towards tertiary alcohols is lowest mainly due to two reasons. First, steric hindrance of the alkyl groups. Second, an increase in the electron density of the Oxygen atom in the hydroxyl bond.
36. What happens when benzene diazonium chloride is heated with water?
Solution:
When benzene diazonium chloride is heated with water, Phenol is formed along with the by-products, Nitrogen gas and Hydrochloric acid.
37. Arrange the following compounds in decreasing order of acidity. H2O, ROH, HC ≡ CH
Solution:
The decreasing order of the acidity of the given compounds:
H2O > ROH > HC ≡ CH
HC ≡ CH is less acidic because the carbon atoms here are sp hybridised, so the electron density is higher on the carbon atom.
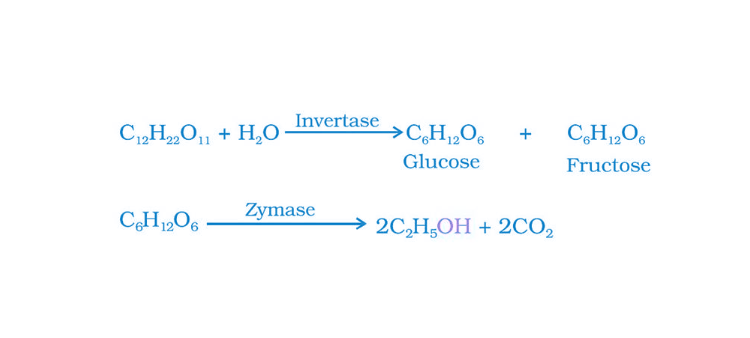
38. Name the enzymes and write the reactions involved in the preparation of ethanol from sucrose by fermentation.
Solution:
The names of the enzymes involved in the preparation of ethanol from sucrose by fermentation are invertase and zymase.
Invertase converts sucrose into glucose and fructose. Then, glucose and fructose undergo fermentation in the presence of zymase, and ethanol is produced.
39. How can propane-2-one be converted into tert- butyl alcohol?
Solution:
Propane-2-one is treated with CH3MgBr in the presence of dry ether (Grignard reagent), followed by hydrolysis to yield tert- butyl alcohol.
40. Write the structures of the isomers of alcohols with molecular formula C4H10O. Which of these exhibits optical activity?
Solution:
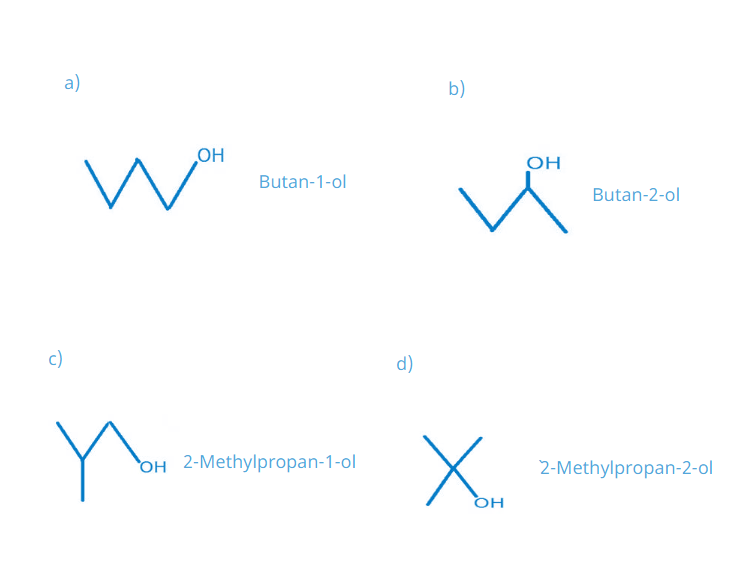
Here, only Butan-2-ol exhibits optical activity because it has a chiral carbon atom.
41. Explain why the OH group in phenols is more strongly held as compared to OH group in alcohols.
Solution:
The –OH group in phenol is directly attached to the sp2hybridized carbon atom of the benzene ring. The carbon-oxygen bond length in phenol is smaller as compared to the carbon-oxygen bond length in alkyl alcohol, and this is due to the partial double bond character or due to the resonance and charge distribution in phenol.
42. Explain why nucleophilic substitution reactions are not very common in phenols.
Solution:
Due to the resonance, the ortho- and para-positions in the benzene ring become electron-rich and, therefore, activates it towards the electrophilic substitution reaction. Thus, nucleophilic substitution reactions are not very common in phenols
43. Preparation of alcohols from alkenes involves the electrophilic attack on an alkene carbon atom. Explain its mechanism.
Solution:
First Step (1) Protonation of alkene and formation of a carbocation

then Step (2) Nucleophilic attack of water
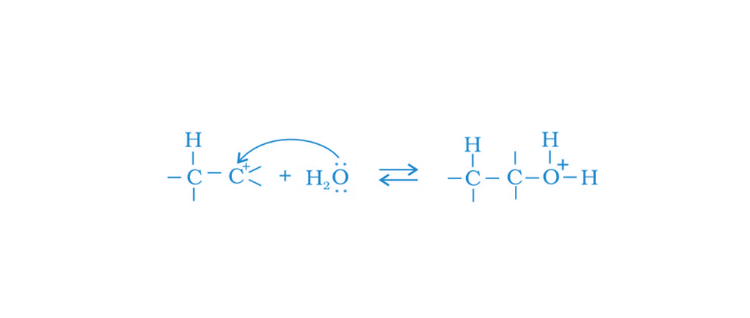
in last Step (3) Deprotonation occurs, and alcohol is formed. H30+ is released.

44. Explain why O=C=O is nonpolar while R—O—R is polar.
Solution:
O=C=O nonpolar because the dipole moment of the two C=O bonds is exactly equal and opposite of each other. Therefore, they cancel each other, and so, the net dipole moment of O=C=O is zero.
45. Why is the reactivity of all three classes of alcohols with conc? Are HCl and ZnCl2 (Lucas reagent) different?
Solution:
This is because of the steric hindrance of the alkyl group and the stability of carbocation.
Primary alcohol does not show any reaction at room temperature because the 1° carbocation is the least stable.
Secondary alcohol does not show any turbidity at room temperature, but on heating, turbidity appears.
Tertiary alcohol immediately shows turbidity after the addition of Lucas reagent as they can form halides easily due to the higher stability of the carbocation.
46. Write steps to carry out the conversion of phenol to aspirin.
Solution:
Phenol is treated with NaOH to produce phenoxide ions. Phenoxide ion then undergoes electrophilic substitution with CO2 to yield salicylic acid as the major product. This is called Kolbe’s reaction.
47. Nitration is an example of aromatic electrophilic substitution, and its rate depends upon the group already present in the benzene ring. Out of benzene and phenol, which one is more easily nitrated and why?
Solution:
Phenol is more nitrated because of the presence of the hydroxyl group in phenol. Due to the resonance effect caused by –OH group, the ortho- and para-positions in the benzene ring becomes electron-rich and, therefore, activates it towards electrophilic substitution reaction. Thus, Nitration, an aromatic electrophilic substitution, occurs at a position where the electron density is high.
48. In Kolbe’s reaction, instead of phenol, phenoxide ion is treated with carbon dioxide. Why?
Solution:
In Kolbe’s reaction, instead of phenol, phenoxide ion is treated with carbon dioxide (a weak electrophile) because phenoxide ion is more reactive towards electrophilic aromatic substitution.
49. The dipole moment of phenol is smaller than that of methanol. Why?
Solution:
The dipole moment of phenol is smaller than that of methanol due to the electron-withdrawing effect of the phenyl ring. Due to the resonance, the polarity of the C-O bond in phenol decreases.
50. Ethers can be prepared by Williamson synthesis, in which an alkyl halide is reacted with sodium alkoxide. Di-tert-butyl ether can’t be prepared by this method. Explain.
Solution:
Ethers can be prepared by Williamson synthesis, in which an alkyl halide is reacted with sodium alkoxide. Di-tert-butyl ether can’t be prepared by this method because in this case, elimination is more favoured over substitution.
51. Why is the C—O—H bond angle in alcohols slightly less than the tetrahedral angle, whereas the C—O—C bond angle in ether is slightly greater?
Solution:
The C—O—H bond angle in alcohols is slightly less than the tetrahedral angle due to the repulsion between the unshared pair of electrons or the lone pair of electrons on the oxygen atom.
52. Explain why low molecular mass alcohols are soluble in water.
Solution:
This is due to the presence of intermolecular hydrogen bonding due to the presence of OH group between alcohol molecules. With an increase in the alkyl group of alcohol or case of high molecular mass alcohols, it suppresses the effect of polar nature of –OH group of alcohol. Thus, the solubility of alcohol decreases with increases in molecular size.
53. Explain why p-nitrophenol is more acidic than phenol.
Solution:
Para-nitrophenol is more acidic than phenol due to the presence of an electron-withdrawing group, -NO2 group, which enhances the acidic strength of the compound by stabilizing the phenoxide ion.
54. Explain why alcohols and ethers of comparable molecular mass have different boiling points?
Solution:
Alcohols and ethers of comparable molecular mass have different boiling points because of the presence of intermolecular hydrogen bonding in alcohols.
55. The carbon-oxygen bond in phenol is slightly stronger than that in methanol. Why?
Solution:
Reason:
i) Due to the resonance, it develops a partial double bond character in the carbon-oxygen bond. Thus, the decrease in the size of the carbon-oxygen bond in phenol.
ii) In phenol, Oxygen is directly attached to a sp2hybridized carbon atom, whereas in methanol, Oxygen is directly attached to an sp3 hybridized carbon atom. Thus, the bond formed between oxygen and the sp2 hybridized carbon atom is slightly stronger than that in methanol.
56. Arrange water, ethanol and phenol in increasing order of acidity and give a reason for your answer.
Solution:
The increasing order of acidity is: Ethanol < water < phenol
Phenol is more acidic because it forms a phenoxide ion after deprotonation and gets stabilized by resonance. Ethanol is less acidic because the ethoxide ion is stabilized by the positive inductive effect. The electron-releasing group in ethanol increases the density of oxygen, and deprotonation gets difficult to occur. Water is a good proton donor than ethanol.
NCERT Exemplar For Class 12 Science
The NCERT exemplars are an effective study material for scoring higher marks in the examination paper. Students must practise these additional questions for their own benefits, as these are curated by the best subject-matter experts to boost both knowledge and confidence. Students can easily access the ncert exemplar for class 12 science by visiting our website SimplyAcad and solve all the questions listed to secure maximum marks.
Here are some other NCERT exemplar for class 12 chemistry:
| NCERT exemplar for class 12 chemistry Chapter 1 | NCERT exemplar for class 12 chemistry Chapter 6 |
|---|---|
| NCERT exemplar for class 12 chemistry Chapter 2 | NCERT exemplar for class 12 chemistry Chapter 7 |
| NCERT exemplar for class 12 chemistry Chapter 3 | NCERT exemplar for class 12 chemistry Chapter 8 |
| NCERT exemplar for class 12 chemistry Chapter 4 | NCERT exemplar for class 12 chemistry Chapter 10 |
| NCERT exemplar for class 12 chemistry Chapter 5 | NCERT exemplar for class 12 chemistry Chapter 12 |
latest video
news via inbox
Nulla turp dis cursus. Integer liberos euismod pretium faucibua