NCERT Exemplar Class 12 Chemistry Chapter 9 Coordination Compounds
NCERT Exemplar for Class 12 Chemistry Chapter 9
The NCERT Exemplar for Class 12 Chemistry is a comprehensive study material prepared by experts to assist students in their study. They will gain deep insights of all the sections and get them ready for the upcoming 12th board examination. As we know, Chemistry requires attention to minor details and information, therefore, by solving exemplars students can recall, memorise, analyse and comprehend better. This is an effective way to increase their confidence and it will result in better performance. The given exemplar contains MCQs of two different types, Short Answer Type Questions, Matching , Assertion and Reason Type questions, there are a total of 45 questions asked. Students can access the NCERT exemplar for class 12 chemistry, Chapter 9: Coordination Compounds by scrolling below. Along with this, there are several NCERT exemplar for class 12 science of all the chapters provided on this platform.
Access Solutions of the NCERT Exemplar Class 12 Chemistry Chapter 9 Coordination Compounds
I. Multiple Choice Questions (Type-I) for Ncert exemplar for class 12 chemistry
1. Which of the following complexes formed by Cu2+ ions is most stable?
| (i) Cu2+ + 4NH3 ⇌ [Cu(NH3)4]2+
(ii) Cu2+ + 4CN– ⇌ [Cu(CN)4]2–, (iii) Cu2+ + 2en ⇌ [Cu(en)2]2+, (iv) Cu2+ + 4H2O⇌ [Cu(H2O)4]2+, |
a) logK
b) logK c) logK d) logK |
11.6
27.3 15.4 8.9 |
|---|
Solution:
Option (ii) is the answer.
2. The colour of the coordination compounds depends on the crystal field splitting. What will be the correct order of absorption of wavelength of light in the visible region, for the complexes, [Co(NH3)6]3+, [Co(CN)6]3–, [Co(H2O)6]3+
(i) [Co(CN)6]3–> [Co(NH3)6]3+>[Co(H2O)6]3+
(ii) [Co(NH3)6]3+> [Co(H2O)6]3+> [Co(CN)6]3–
(iii) [Co(H2O)6]3+> [Co(NH3)6]3+> [Co(CN)6]3–
(iv) [Co(CN)6]3–> [Co(NH3)6]3+> [Co(H2O)6]3+
Solution:
Option (iii) is the answer.
3. When 0.1 mol CoCl3(NH3)5 is treated with an excess of AgNO3, 0.2 mol of AgCl are obtained. The conductivity of the solution will correspond to
(i) 1:3 electrolyte
(ii) 1:2 electrolyte
(iii) 1:1 electrolyte
(iv) 3:1 electrolyte
Solution:
Option (ii) is the answer.
4. When 1 mol CrCl3.6H2O is treated with an excess of AgNO3, 3 mol of AgCl are obtained. The formula of the complex is :
(i) [CrCl3 (H2O)3].3H2O
(ii) [CrCl2(H2O)4]Cl.2H2O
(iii) [CrCl(H2O)5]Cl2.H2O
(iv) [Cr(H2O)6]Cl3
Solution:
Option (iv) is the answer.
5. The correct IUPAC name of [Pt(NH3)2Cl2] is
(i) Diamminedichloridoplatinum (II)
(ii) Diamminedichloridoplatinum (IV)
(iii) Diamminedichloridoplatinum (0)
(iv) Dichloridodiammineplatinum (IV)
Solution:
Option (i) is the answer.
6. The stabilisation of coordination compounds due to chelation is called the chelate effect. Which of the following is the most stable complex species?
(i) [Fe(CO)5]
(ii) [Fe(CN)6]3–
(iii) [Fe(C2O4)3]3–
(iv) [Fe(H2O)6]3+
Solution:
Option (iii) is the answer.
7. Indicate the complex ion which shows geometrical isomerism.
(i) [Cr(H2O)4Cl2]+
(ii) [Pt(NH3)3 Cl]
(iii) [Co(NH3)6]3+
(iv) [Co(CN)5(NC)]3–
Solution:
Option (i) is the answer.
8. The CFSE for octahedral [CoCl6]4– is 18,000 cm–1. The CFSE for tetrahedral [CoCl4]2– will be
(i) 18,000 cm–1
(ii) 16,000 cm–1
(iii) 8,000 cm–1
(iv) 20,000 cm–1
Solution:
Option (iii) is the answer.
9. Due to the presence of ambidentate ligands coordination compounds show isomerism. Palladium complexes of the type [Pd(C6H5)2(SCN)2] and [Pd(C6H5)2(NCS)2] are
(i) linkage isomers
(ii) coordination isomers
(iii) ionisation isomers
(iv) geometrical isomers
Solution:
Option (i) is the answer.
10. The compounds [Co(SO4)(NH3)5]Br and [Co(SO4)(NH3)5]Cl represent
(i) linkage isomerism
(ii) ionisation isomerism
(iii) coordination isomerism
(iv) no isomerism
Solution:
Option (iv) is the answer.
11. A chelating agent has two or more than two donor atoms to bind to a single metal ion. Which of the following is not a chelating agent?
(i) thiosulphate
(ii) oxalate
(iii) glycinato
(iv) ethane-1,2-diamine
Solution:
Option (i) is the answer.
12. Which of the following species is not expected to be a ligand?
(i) NO
(ii) NH4+
(iii) NH2CH2CH2NH2
(iv) CO
Solution:
Option (ii) is the answer.
13. What kind of isomerism exists between [Cr(H2O)6]Cl3 (violet) and [Cr(H2O)5Cl]Cl2.H2O (greyish-green)?
(i) linkage isomerism
(ii) solvate isomerism
(iii) ionisation isomerism
(iv) coordination isomerism
Solution:
Option (ii) is the answer.
14. IUPAC name of [Pt (NH3)2Cl(NO2)] is :
A. Platinum diaminechloronitrite
B. Chloronitrito-N-ammineplatinum (II)
C. Diamminechloridonitrito-N-platinum (II)
D. Diamminechloronitrito-N-platinate (II)
Solution:
Option (iii) is the answer.
II. Multiple Choice Questions (Type-II)
Note: In the following questions two or more options may be correct.
15. The atomic number of Mn, Fe and Co are 25, 26 and 27 respectively. Which of the following inner orbital octahedral complexions are diamagnetic?
(i) [Co(NH3)6]3+
(ii) [Mn(CN)6]3–
(iii) [Fe(CN)6]4–
(iv) [Fe(CN)6]3–
Solution:
Option (i) and (iii) are the answers.
16. The atomic number of Mn, Fe, Co and Ni are 25, 26 27 and 28 respectively. Which of the following outer orbital octahedral complexes have the same number of unpaired electrons?
(i) [MnCl6]3–
(ii) [FeF6]3–
(iii) [CoF6]3–
(iv) [Ni(NH3)6]2+
Solution:
Option (i) and (iii) is the answer.
17. Which of the following options are correct for [Fe(CN)6]3– complex?
(i) d2sp3hybridisation
(ii) sp3d2hybridisation
(iii) paramagnetic
(iv) diamagnetic
Solution;
Option (i) and (iii) are the answers.
18. An aqueous pink solution of cobalt(II) chloride changes to deep blue on the addition of an excess of HCl. This is because____________.
(i) [Co(H2O)6]2+ is transformed into [CoCl6]4–
(ii) [Co(H2O)6]2+ is transformed into [CoCl4]2–
(iii) tetrahedral complexes have smaller crystal field splitting than octahedral complexes.
(iv) tetrahedral complexes have larger crystal field splitting than octahedral complex.
Solution:
Option (ii) and (iii) are the answers.
19. Which of the following complexes is homoleptic?
(i) [Co(NH3)6]3+
(ii) [Co(NH3)4 Cl2]+
(iii) [Ni(CN)4]2–
(iv) [Ni(NH3)4Cl2]
Solution:
Option (i) and (iii) are the answers.
20. Which of the following complexes are heteroleptic?
(i) [Cr(NH3)6]3+
(ii) [Fe(NH3)4 Cl2]+
(iii) [Mn(CN)6]4–
(iv) [Co(NH3)4Cl2]
Solution:
Option (ii) and (iv) are the answers.
21. Identify the optically active compounds from the following :
(i) [Co(en)3]3+
(ii) trans– [Co(en)2 Cl2]+
(iii) cis– [Co(en)2 Cl2]+
(iv) [Cr (NH3)5Cl]
Solution:
Option (i) and (iii) are the answers.
22. Identify the correct statements for the behaviour of ethane-1, 2-diamine as a ligand.
(i) It is a neutral ligand.
(ii) It is a didentate ligand.
(iii) It is a chelating ligand.
(iv) It is a unidentate ligand.
Solution:
Option (i), (ii) and (iii) are the answers.
23. Which of the following complexes show linkage isomerism?
(i) [Co(NH3)5 (NO2)]2+
(ii) [Co(H2O)5CO]3+
(iii) [Cr(NH3)5 SCN]2+
(iv) [Fe(en)2 Cl2]+
Solution:
Option (i) and (iii) are the answers.
III. Short Answer Type for Ncert exemplar for class 12 chemistry
24. Arrange the following complexes in the increasing order of conductivity of their solution: [Co(NH3)3Cl3], [Co(NH3)4Cl2] Cl, [Co(NH3)6]Cl3 , [Cr(NH3)5Cl]Cl2
Solution:
The increasing order of conductivity is as follows:
[Co(NH3)3Cl3]<[Co(NH3)4Cl2]Cl< [Cr(NH3)5Cl]Cl2<[Co(NH3)6]Cl325. A coordination compound CrCl3.4H2O precipitates silver chloride when treated with silver nitrate. The molar conductance of its solution corresponds to a total of two ions. Write the structural formula of the compound and name it.
Solution:
If it forms silver chloride then there is one free chlorine atom outside the coordination sphere. The structural formula has to be [Cr(H2O)4Cl2]Cl. The name of this complex is tetraaquadichlorido chromium(III) chloride.
26. A complex of the type [M(AA)2X2]n+is known to be optically active. What does this indicate about the structure of the complex? Give one example of such complex.
Solution:
The structure has to be cis-octahedral.
Example for such a complex is [Co(en)2Cl2]+ which is optically active.
27. The magnetic moment of [MnCl4]2– is 5.92 BM. Explain giving a reason.
Solution:
A magnetic moment of 5.92 BM means there are 5 unpaired electrons because
Magnetic Moment = √ n(n+2)
The geometry tetrahedral with 5 unpaired electrons giving a magnetic moment of 5.92 BM as the four ligands are attached to Mn2+.
28. Based on crystal field theory explain why Co(III) forms a paramagnetic octahedral complex with weak field ligands whereas it forms a diamagnetic octahedral complex with strong field ligands.
Solution:
The electronic configuration will be t42g e2g. It has 4 unpaired electron and paramagnetic. With weal ligand Δ0 < p. The configuration with strong field ligand will be t62g e0g. the Δ0 > p and there won’t be any unpaired electron therefore diamagnetic.
29. Why are low spin tetrahedral complexes not formed?
Solution:
For tetrahedral complexes, the crystal field splitting energy is too low. It is lower than pairing energy so, the pairing of electrons is not favoured and therefore the complexes cannot form low spin complexes.
30. Give the electronic configuration of the following complexes based on Crystal Field Splitting theory. [CoF6]3–, [Fe(CN)6]4– and [Cu(NH3)6]2+.
Solution:
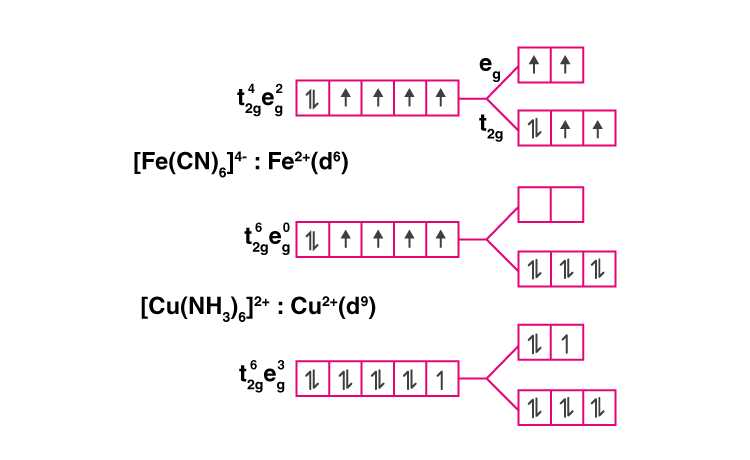
31. Explain why [Fe(H2O)6]3+ has a magnetic moment value of 5.92 BM whereas [Fe(CN)6]3– has a value of only 1.74 BM.
Solution:
For [Fe(H2O)6]3+, H2O is a weak field ligand won’t cause pairing of electrons. So, the number of unpaired electrons will be 5.
[Fe(CN)6]3–, Fe3+ has six unpaired electrons. CN- is a strong field ligand which will cause pairing of all the electrons. So, the electrons will start pairing leaving behind one unpaired electron.32. Arrange following complex ions in increasing order of crystal field splitting energy (DO) : [Cr(Cl)6]3–, [Cr(CN)6]3–, [Cr(NH3)6]3+.
Solution:
The increasing order of crystal field energy is
[Cr(Cl)6]3–<[Cr(NH3)6]3+ <[Cr(CN)6]3–This is also the order of field strength of the ligands according to the spectrochemical series.
33. Why do compounds having similar geometry have a different magnetic moment?
Solution:
They differ in the number of paired and unpaired electrons. A strong field ligand will cause pairing of electrons while a weak field ligand will not cause pairing. Pairing or not pairing will change the number of unpaired electrons, which affects the magnetic moment.
34. CuSO4.5H2O is blue while CuSO4 is colourless. Why?
Solution:
In CuSO4.5H2O, there are water molecules that act as ligands. The electrons will excite to higher d orbital and show colour. Whereas, in CuSO4, there are no water molecules to act as ligands, so no crystal field splitting happens.
35. Name the type of isomerism when ambidentate ligands are attached to a central metal ion. Give two examples of ambidentate ligands.
Solution:
Ambidendate ligands are those having different two binding sites.
Examples: Isothiocyanato Thiocyanato and Nitrite-N Nitrito-O
The type of isomerism when ambidentate ligands are attached to a central metal ion is called linkage isomerism because they only differ in the atom that is linked to the central metal ion.
IV. Matching Type
Note: In the following questions match the items given in Columns I and II.
36. Match the complex ions given in Column I with the colours given in Column II and assign the correct code :
| Column I (Complex ion)
A. [Co(NH3)6]3+ B. [Ti(H2O)6]3+ C. [Ni(H2O)6]2+ D. (Ni (H2O)4(en)]2+ (aq) |
Column II (Colour)
1. Violet 2. Green 3. Pale blue 4. Yellowish orange 5. Blue |
|---|
Code :
(i) A (1) B (2) C (4) D (5)
(ii) A (4) B (3) C (2) D (1)
(iii) A (3) B (2) C (4) D (1)
(iv) A (4) B (1) C (2) D (3)
Solution:
Option (ii) is the answer.
37. Match the coordination compounds given in Column I with the central metal atoms given in Column II and assign the correct code :
| Column I (Coordination Compound)
A. Chlorophyll B. Blood pigment C. Wilkinson catalyst D. Vitamin B12 |
Column II (Central metal atom)
1. rhodium 2. cobalt 3. calcium 4. iron 5. magnesium |
|---|
Code :
(i) A (5) B (4) C (1) D (2)
(ii) A (3) B (4) C (5) D (1)
(iii) A (4) B (3) C (2) D (1)
(iv) A (3) B (4) C (1) D (2)
Solution:
Option (i) is the answer.
38. Match the complex ions given in Column I with the hybridisation and number of unpaired electrons given in Column II and assign the correct code :
| Column I (Complex ion)
A. [Cr(H2O)6]3+ B. [Co(CN)4]2– 2. C. [Ni(NH3)6]2+ D. [MnF6]4– |
Column II (Hybridisation,
number of unpaired electrons) 1. dsp2, 1 2.sp3d2, 5 3. d2sp3, 3 4. sp3, 4 5. sp3d2, 2 |
|---|
Code :
(i) A (3) B (1) C (5) D (2)
(ii) A (4) B (3) C (2) D (1)
(iii) A (3) B (2) C (4) D (1)
(iv) A (4) B (1) C (2) D (3)
Solution:
Option (i) is the answer
39. Match the complex species given in Column I with the possible isomerism given in Column II and assign the correct code :
| Column I (Complex species)
A. [Co(NH3)4Cl2]+ B. cis-[Co(en)2Cl2]+ C. [Co(NH3)5(NO2)]Cl2 D. [Co(NH3)6][Cr(CN)6] |
Column II (Isomerism)
1. Optical 2. Ionisation 3. Coordination 4. Geometrical 5. Linkage |
|---|
Code :
(i) A (1) B (2) C (4) D (5)
(ii) A (4) B (3) C (2) D (1)
(iii) A (4) B (1) C (5) D (3)
(iv) A (4) B (1) C (2) D (3)
Solution:
Option (iv) is the answer
40. Match the compounds given in Column I with the oxidation state of cobalt present in it (given in Column II) and assign the correct code.
| Column I (Compound)
A. [Co(NCS)(NH3)5](SO3) B. [Co(NH3)4Cl2]SO4 C. Na4[Co(S2O3)3] D. [Co2(CO)8] |
Column II (Oxidation state of Co)
1.+4 2. 0 3. +1 4.+2 5. +3 |
|---|
Code :
(i) A (1) B (2) C (4) D (5)
(ii) A (4) B (3) C (2) D (1)
(iii) A (5) B (1) C (4) D (2)
(iv) A (4) B (1) C (2) D (3)
Solution:
Option (ii) is the answer.
V. Assertion and Reason Type
Note: In the following questions a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
(i) Assertion and reason both are true, the reason is the correct explanation of assertion.
(ii) Assertion and reason both are true but the reason is not the correct explanation of assertion.
(iii) An assertion is true, the reason is false.
(iv) The assertion is false, the reason is true.
41. Assertion: Toxic metal ions are removed by the chelating ligands. Reason: Chelate complexes tend to be more stable.
Solution:
Option (i) is correct.
42. Assertion : [Cr(H2O)6]Cl2 and [Fe(H2O)6]Cl2 are reducing in nature. Reason: Unpaired electrons are present in their d-orbitals.
Solution:
Option (ii) is correct.
43. Assertion: Linkage isomerism arises in coordination compounds containing ambidentate ligand. Reason: Ambidentate ligand has two different donor atoms.
Solution:
Option (i) is correct
44. Assertion: Complexes of MX6 and MX5L type (X and L are unidentate) do not show geometrical isomerism. Reason: Geometrical isomerism is not shown by complexes of coordination number 6.
Solution:
Option (iii) is correct.
45. Assertion : ([Fe(CN)6]3– ion shows magnetic moment corresponding to two unpaired electrons.
Reason: Because it has d2sp3 type hybridisation.
Solution:
Option (iv) is correct.
NCERT Exemplar For Class 12 Science
The NCERT exemplars are an effective study material for scoring higher marks in the examination paper. Students must practise these additional questions for their own benefits, as these are curated by the best subject-matter experts to boost both knowledge and confidence. Students can easily access the ncert exemplar for class 12 science by visiting our website SimplyAcad and solve all the questions listed to secure maximum marks.
Here are some other NCERT exemplar for class 12 chemistry:
| NCERT exemplar for class 12 chemistry Chapter 1 | NCERT exemplar for class 12 chemistry Chapter 7 |
|---|---|
| NCERT exemplar for class 12 chemistry Chapter 3 | NCERT exemplar for class 12 chemistry Chapter 8 |
| NCERT exemplar for class 12 chemistry Chapter 4 | NCERT exemplar for class 12 chemistry Chapter 10 |
| NCERT exemplar for class 12 chemistry Chapter 5 | NCERT exemplar for class 12 chemistry Chapter 11 |
| NCERT exemplar for class 12 chemistry Chapter 6 | NCERT exemplar for class 12 chemistry Chapter 12 |
latest video
news via inbox
Nulla turp dis cursus. Integer liberos euismod pretium faucibua