NCERT Solutions for Class 8 Science Chapter 6 Combustion and Flame
Combustion And Flame NCERT Solutions Class 8

Combustion And Flame is an essential chapter for class 8 students and they must prepare it thoroughly to do well in their exams. Students struggling with the questions can access the provided NCERT Solutions for Class 8 Science Combustion And Flame by SimplyAcad. The answers are explained in a detailed and comprehensive manner and aid learners with understanding of the difficult topics the chapter constitutes.
NCERT Solutions for Class 8 Science of Combustion And Flame can be easily availed to students by scrolling below.
Access NCERT Solutions of Combustion And Flame
1. List conditions under which combustion can take place.
Soln:
The burning of a substance in the presence of oxygen is defined as combustion.
The conditions under which combustion can take place are
- The presence of air or oxygen.
- The presence of fuel plays a significant role.
- Ignition temperature is maintained (It is defined as the substance that catches fire at its lowest temperature.)
2. Fill in the blanks.
(a) Burning of wood and coal causes __________of air.
(b) A liquid fuel, used in homes is__________ .
(c) Fuel must be heated to its ____________ before it starts burning.
(d) The fire produced by oil cannot be controlled by___________ .
Soln:
(a) Burning of wood and coal causes pollution of air.
(b) A liquid fuel, used in homes is kerosene.
(c) Fuel must be heated to its ignition temperature before it starts burning.
(d) The fire produced by oil cannot be controlled by water.
3. Explain how the use of CNG in automobiles has reduced pollution in our cities.
Soln:
CNG plays an important role in reducing pollution among automobiles for the following reasons:
- CNG is comparatively a cleaner fuel.
- The CNG can be an alternative for diesel, petrol and propane/LPG.
- It usually contains a few undesirable gases than the other fuels mentioned above.
- The combustion of fuels like petroleum causes many unburnt carbon particles along with carbon monoxide, which leads to respiratory diseases.
4. Compare LPG and wood as fuels.
Soln:
Wood
- It is considered as a traditional fuel used for both domestic and industrial purposes.
- Wood produces a lot of smoke which pollutes the atmosphere and causes respiratory diseases.
- The usage of wood to a large extent causes deforestation.
- The calorific value of wood ranges between 17000 and 22000 kJ/kg
- However, wood may be used as a furnace, stove or fireplace indoors while it is used for a campfire, furnace outdoors.
LPG
- The usage LPG (Liquefied petroleum gas) has replaced wood.
- It doesn’t release smoke and other pollutants.
- It is a cleaner fuel.
- The fuel efficiency of LPG is more than that of wood.
- The calorific value of LPG is 55000 kJ/kg.
- Hence, LPG is the most preferred choice.
5. Give reasons.
(a) Water is not used to control fires involving electrical equipment.
(b) LPG is a better domestic fuel than wood.
(c) Paper by itself catches fire easily whereas a piece of paper wrapped around an aluminium pipe does not.
Soln:
a)
- Water is a good conductor of electricity.
- If added to an electrical fire, the water would just spread the electricity further.
- The person dousing the fire might get an electric shock.
b)
- LPG, being a cleaner fuel than wood, doesn’t release smoke and other pollutants.
- Wood, on the other hand, releases a lot of smoke and fumes polluting the atmosphere causing pollution and leading to respiratory diseases.
- Hence, LPG is a better domestic fuel than wood.
c)
- The paper by itself catches fire easily because of its low ignition temperature.
- The piece of paper wrapped around an aluminium pipe doesn’t catch fire because aluminium is a good conductor of electricity.
- While the paper wrapped around an aluminium pipe results in an increase in ignition temperature. So, there is a transfer of heat from paper to the aluminium pipe. Hence, it doesn’t catch fire.
6. Make a labelled diagram of a candle flame.
Soln:
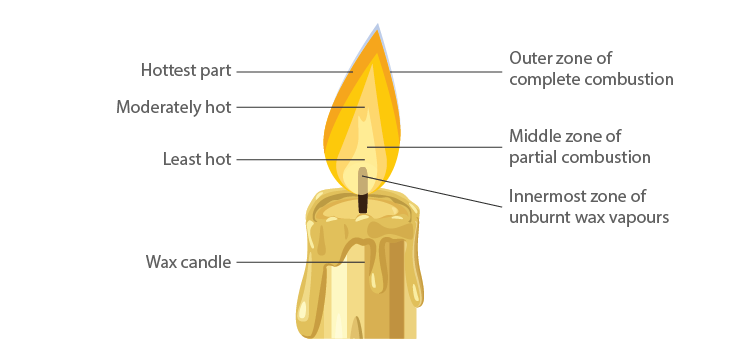
7. Name the unit in which the calorific value of a fuel is expressed.
Soln:
Calorific value is defined as the energy contained in the fuel. It is expressed in the form kJ/kg
kJ=kilo joules and kg=kilogram
8. Explain how CO2 is able to control fires.
Soln:
CO2 is a non-combustible gas and extinguishes fire in two ways:
(i) It is heavier than oxygen and covers the fire like a blanket and cuts off the contact between oxygen and fuel.
(ii) In cylinders, CO2 is kept in the liquid form. When released, it expands enormously. This brings down the temperature of the fuel, which helps in controlling the fire.
9. It is difficult to burn a heap of green leaves but dry leaves catch fire easily. Explain.
Soln:
A heap of green leaves contains a lot of moisture in it, hence its ignition temperature is high. Therefore, it does not catch fire easily.
But dry leaves have no moisture content in them, hence their ignition temperature is low. Therefore, they catch fire easily.
10. Which zone of a flame does a goldsmith use for melting gold and silver and why?
Soln:
A goldsmith mainly uses non-luminous flame which is considered to be the outermost part of the flame. This part of the flame is used because the outermost flame undergoes complete combustion and is considered as the hottest part of the flame.
11. In an experiment, 4.5 kg of fuel was burnt completely. The heat produced was measured to be 180,000 kJ. Calculate the calorific value of the fuel. \section*
{Solution} \textbf{Calorific value:} The amount of heat energy released on complete combustion of 1 kg of fuel is called the calorific value. It is expressed in a unit called kilojoule per kg (kJ/kg). \begin{align*} \text{Calorific value} &= \frac{\text{Heat produced}}{\text{Weight of fuel used in kg}} \\ \text{Heat produced} &= 180,000 \, \text{kJ} \\ \text{Weight of the fuel used} &= 4.5 \, \text{kg} \\ \text{Calorific value} &= \frac{180,000 \, \text{kJ}}{4.5 \, \text{kg}} \\ &= 40,000 \, \text{kJ/kg} \end{align*}
12. Can the process of rusting be called combustion? Discuss.
Soln:
No, because rusting is an exothermic process as heat is liberated during rusting. On the other hand, combustion is a chemical process in which a substance reacts with oxygen to release energy in the form of heat or light.
13. Abida and Ramesh were doing an experiment in which water was to be heated in a beaker. Abida kept the beaker near the wick in the yellow part of the candle flame. Ramesh kept the beaker in the outermost part of the flame. Whose water will get heated in a shorter time?
Soln:
The water placed in the outermost part of the flame will be heated in a short time since it is a non-luminous flame and is regarded as the hottest part of the flame. So Ramesh’s beaker will be heated first. However, Abida who placed the beaker in the luminous flame (yellow flame) is comparatively less hot.
latest video
news via inbox
Nulla turp dis cursus. Integer liberos euismod pretium faucibua