NCERT Solutions for Class 12 Chemistry Chapter 6 Haloalkanes and Haloarenes
Haloalkanes and Haloarenes Chapter 6: NCERT Solutions for Class 12
NCERT Solutions for Class 12 Haloalkanes and Haloarenes Chapter 6 is provided here for students to gain insight on the several topics of the chapter. The solutions will allow students to learn the basic concepts of Haloalkanes and Haloarenes, their functions and importance in chemical reactions.
Experts prepare the best exercises to test the students’ understanding, simultaneously, several question and sample papers mostly contain problems from the exercises given in your NCERT textbooks , therefore, solving them is essential for every student. Completing exercises of the textbook help students to master each portion of the chapter well. Scroll below to find the answers provided below by SimplyAcad.

NCERT Solutions for Class 12 Haloalkanes and Haloarenes Chapter 6
Question 1. Name the following halides according to IUPAC system and classify them as alkyl, allyl, benzyl (primary, secondary, tertiary), vinyl or aryl halides:
(i) (CH3)2CHCH(Cl)CH3
(ii) CH3CH2CH(CH3)CH(C2H5)Cl
(iii) CH3CH2C(CH3)2CH2I
(iv) (CH3)3CCH2CH(Br)C6H5
(v) CH3CH(CH3)CH(Br)CH3
(vi) CH3C(C2H5)2CH2Br
(vii) CH3C(Cl)(C2H5)CH2CH3
(viii) CH3CH=C(Cl)CH2CH(CH3)2
(ix) CH3CH=CHC(Br)(CH3)2
(x) p-ClC6H4CH2CH(CH3)2
(xi) m-ClCH2C6H4CH2C(CH3)3
(xii) o-Br-C6H4CH(CH3)CH2CH3
Solution :
(i)
2−Chloro−3−methylbutane
(Secondary alkyl halide)
(ii)

3−Chloro−4−methyhexane
(Secondary alkyl halide)
(iii)
1−Iodo−2, 2 −dimethylbutane
(Primary alkyl halide)
(iv)

1−Bromo−3, 3−dimethyl−1−phenylbutane
(Secondary benzyl halide)
(v)
2−Bromo−3−methylbutane
(Secondary alkyl halide)
(vi)
1−Bromo−2−ethyl−2−methylbutane
(Primary alkyl halide)
(vii)
3−Chloro−3−methylpentane
(Tertiary alkyl halide)
(viii)

3−Chloro−5−methylhex−2−ene
(Vinyl halide)
(ix)
4−Bromo−4−methylpent−2−ene
(Allyl halide)
(x)
1−Chloro−4−(2−methylpropyl) benzene
(Aryl halide)
(xi)
1−Chloromethyl−3−(2, 2−dimethylpropyl) benzene
(Primary benzyl halide)
(xii)
1−Bromo−2−(1−methylpropyl) benzene
(Aryl halide)
Question2. Give the IUPAC names of the following compounds:
(i) CH3CH(Cl)CH(Br)CH3
(ii) CHF2CBrClF
(iii) ClCH2C≡CCH2Br
(iv) (CCl3)3CCl
(v) CH3C(p-ClC6H4)2CH(Br)CH3
(vi) (CH3)3CCH=CClC6H4I-p
Solution :
(i)
2−Bromo−3−chlorobutane
(ii)
1−Bromo−1−chloro−1, 2, 2−trifluoroethane
(iii)
![]()
1−Bromo−4−chlorobut−2−yne
(iv)
2−(Trichloromethyl)−1,1,1,2,3,3,3−heptachloropropane
(v)

2−Bromo−3, 3−bis(4−chlorophenyl) butane
(vi)
1−chloro−1−(4−iodophenyl)−3, 3−dimethylbut−1−ene
Question3. Write the structures of the following organic halogen compounds.
(i) 2-Chloro-3-methylpentane
(ii) p-Bromochlorobenzene
(iii) 1-Chloro-4-ethylcyclohexane
(iv) 2-(2-Chlorophenyl)-1-iodooctane
(v) Perfluorobenzene
(vi) 4-tert-Butyl-3-iodoheptane
(vii) 1-Bromo-4-sec-butyl-2-methylbenzene
(viii) 1,4-Dibromobut-2-ene
Solution :
(i)
2-Chloro-3-methylpentane
(ii)
p-Bromochlorobenzene
(iii)
1-Chloro-4-ethylcyclohexane
(iv)

2-(2-Chlorophenyl)-1-iodooctane
(v)
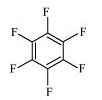
Perfluorobenzene
(vi)

4-Tert-Butyl-3-iodoheptane
(vii)
1-Bromo-4-sec-butyl-2-methylbenzene
(viii)
![]()
1,4-Dibromobut-2-ene
Question4. Which one of the following has the highest dipole moment?
(i) CH2Cl2
(ii) CHCl3
(iii) CCl4
Solution :
(i)
Dichlormethane (CH2Cl2)
μ = 1.60D
(ii)
Chloroform (CHCl3)
μ = 1.08D
(iii)
Carbon tetrachloride (CCl4)
μ = 0D
CCl4 is a symmetrical molecule. Therefore, the dipole moments of all four C−Cl bonds cancel each other. Hence, its resultant dipole moment is zero.
As shown in the above figure, in CHCl3, the resultant of dipole moments of two C−Cl bonds is opposed by the resultant of dipole moments of one C−H bond and one C−Cl bond. Since the resultant of one C−H bond and one C−Cl bond dipole moments is smaller than two C−Cl bonds, the opposition is to a small extent. As a result, CHCl3 has a small dipole moment of 1.08 D.
On the other hand, in the case of CH2Cl2, the resultant of the dipole moments of two C−Cl bonds is strengthened by the resultant of the dipole moments of two C−H bonds. As a result, CH2Cl2 has a higher dipole moment of 1.60 D than CHCl3 i.e., CH2Cl2 has the highest dipole moment.
Hence, the given compounds can be arranged in the increasing order of their dipole moments as:
CCl4 < CHCl3 < CH2Cl2
Question5. A hydrocarbon \(C_5H_{10}\) does not react with chlorine in the dark but gives a single monochloro compound \(C_5H_9Cl\) in bright sunlight. Identify the hydrocarbon. \section*
{Solution} The molecular formula of the hydrocarbon is \(C_5H_{10}\), which satisfies the general formula \(C_nH_{2n}\). This suggests that the hydrocarbon is either an alkene or a cycloalkane. The fact that it does not react with chlorine in the dark indicates that it is not an alkene, as alkenes typically react with chlorine in the dark. Instead, this behavior is characteristic of cycloalkanes. When exposed to bright sunlight, the hydrocarbon gives a single monochloro compound \(C_5H_9Cl\). This suggests that the hydrocarbon is a cycloalkane, specifically cyclopentane, since cyclopentane reacts with chlorine under UV light to yield a single monochloro derivative. Thus, the hydrocarbon is cyclopentane.
Question 6. Write the isomers of the compound having formula C4H9Br. : Haloalkanes and Haloarenes Chapter 6
Solution :
There are four isomers of the compound having the formula C4H9Br. These isomers are given below.
(a)
1−Bromobutane
(b)
2−Bromobutane
(c)
1−Bromo−2−methylpropane
(d)
2−Bromo−2−methylpropane
Question 7. Write the equations for the preparation of 1−iodobutane from
(i) 1-butanol
(ii) 1-chlorobutane
(iii) but-1-ene.
Solution :
(i)
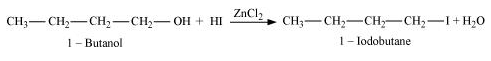
(ii)

(iii) 
Question 8. What are ambident nucleophiles? Explain with an example. \section*
{Solution} Ambident nucleophiles are nucleophiles that can attack an electrophile through two different sites. This duality in the nucleophilic centers allows them to form different products depending on which site is involved in the reaction. An example of an ambident nucleophile is the cyanide ion (\(\text{CN}^-\)). The cyanide ion can attack an electrophile through either the carbon atom or the nitrogen atom, leading to different types of products: 1. **Carbon Attack**: When the cyanide ion attacks through the carbon atom, it forms an alkyl cyanide (also known as a nitrile). For example: \[ \text{R-X} + \text{CN}^- \rightarrow \text{R-CN} + \text{X}^- \] where \(\text{R-X}\) is an alkyl halide. 2. **Nitrogen Attack**: When the cyanide ion attacks through the nitrogen atom, it forms an alkyl isocyanide (also known as an isonitrile). For example: \[ \text{R-X} + \text{NC}^- \rightarrow \text{R-NC} + \text{X}^- \] Thus, the cyanide ion’s ability to attack through both carbon and nitrogen atoms illustrates its ambident nature.
Question 9. Which compound in each of the following pairs will react faster in an \(\text{S}_\text{N}2\) reaction with \(\text{OH}^-\)? \begin{enumerate} \item \(\text{CH}_3\text{Br}\)\text{or}\(\text{CH}_3\text{I}\)\item\((\text{CH}_3)_3\text{CCl}\) \text{ or } \(\text{CH}_3\text{Cl}\) \end{enumerate} \section*
{Solution} 1. **\(\text{CH}_3\text{I}\) will react faster** because the bond dissociation enthalpy of the \(\text{C-I}\) bond is lower than that of the \(\text{C-Br}\) bond. This makes the \(\text{C-I}\) bond weaker and easier to break, facilitating a faster \( \text{S}_\text{N}2 \) reaction. 2. **\(\text{CH}_3\text{Cl}\) will react faster** due to less steric hindrance compared to \((\text{CH}_3)_3\text{CCl}\). In \( \text{S}_\text{N}2 \) reactions, the nucleophile attacks the carbon atom directly. Less steric hindrance around the carbon makes it easier for the nucleophile to approach and react.
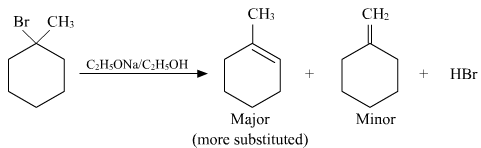
Question 10. Predict all the alkenes that would be formed by dehydrohalogenation of the following halides with sodium ethoxide in ethanol and identify the major alkene:
(i) 1-Bromo-1-methylcyclohexane
(ii) 2-Chloro-2-methylbutane
(iii) 2,2,3-Trimethyl-3-bromopentane.
Solution :
(i)
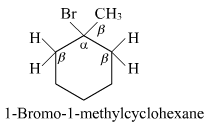
In the given compound, there are two types of β-hydrogen atoms are present. Thus, dehydrohalogenation of this compound gives two alkenes.
(ii)
In the given compound, there are two different sets of equivalent β-hydrogen atoms labelled as a and b. Thus, dehydrohalogenation of the compound yields two alkenes.

Saytzeff’s rule implies that in dehydrohalogenation reactions, the alkene having a greater number of alkyl groups attached to a doubly bonded carbon atoms is preferably produced.
Therefore, alkene (I) i.e., 2-methylbut-2-ene is the major product in this reaction.
(iii)
2,2,3-Trimethyl-3-bromopentane
In the given compound, there are two different sets of equivalent β-hydrogen atoms labelled as a and b. Thus, dehydrohalogenation of the compound yields two alkenes.

According to Saytzeff’s rule, in dehydrohalogenation reactions, the alkene having a greater number of alkyl groups attached to the doubly bonded carbon atom is preferably formed.
Hence, alkene (I) i.e., 3,4,4-trimethylpent-2-ene is the major product in this reaction.
Question13. Give the uses of freon 12, DDT, carbon tetrachloride and iodoform. : Haloalkanes and Haloarenes Chapter 6
Solution :
Uses of Freon − 12
Freon-12 (dichlorodifluoromethane, CF2Cl2) is commonly known as CFC. It is used as a refrigerant in refrigerators and air conditioners. It is also used in aerosol spray propellants such as body sprays, hair sprays, etc. However, it damages the ozone layer. Hence, its manufacture was banned in the United States and many other countries in 1994.
Uses of DDT
DDT (p, p−dichlorodiphenyltrichloroethane) is one of the best known insecticides. It is very effective against mosquitoes and lice. But due its harmful effects, it was banned in the United States in 1973.
Uses of carbontetrachloride (CCl4)
(i) It is used for manufacturing refrigerants and propellants for aerosol cans.
(ii) It is used as feedstock in the synthesis of chlorofluorocarbons and other chemicals.
(iii) It is used as a solvent in the manufacture of pharmaceutical products.
(iv) Until the mid 1960’s, carbon tetrachloride was widely used as a cleaning fluid, a degreasing agent in industries, a spot reamer in homes, and a fire extinguisher.
Uses of iodoform (CHI3)
Iodoform was used earlier as an antiseptic, but now it has been replaced by other formulations-containing iodine-due to its objectionable smell. The antiseptic property of iodoform is only due to the liberation of free iodine when it comes in contact with the skin.
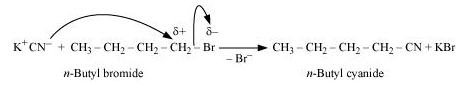
Question15. Write the mechanism of the following reaction:
![]()
Solution :
The given reaction is:
![]()
The given reaction is an SN2 reaction. In this reaction, CN− acts as the nucleophile and attacks the carbon atom to which Br is attached. CN− ion is an ambident nucleophile and can attack through both C and N. In this case, it attacks through the C-atom.
Question16. Arrange the compounds of each set in order of reactivity towards $S_N2$ displacement. \begin{enumerate} \item 2-Bromo-2-methylbutane, 1-Bromopentane, 2-Bromopentane \item 1-Bromo-3-methylbutane, 2-Bromo-2-methylbutane, 2-Bromo-3-methylbutane \item 1-Bromobutane, 1-Bromo-2,2-dimethylpropane, 1-Bromo-2-methylbutane, 1-Bromo-3-methylbutane \end{enumerate} \section*
{Solution} In $S_N2$ reactions, steric factors are crucial. The reactivity of alkyl halides in $S_N2$ reactions is inversely related to steric hindrance around the carbon bearing the leaving group. The general order of reactivity is: \[ 1^\circ > 2^\circ > 3^\circ \] Thus, the reactivity towards $S_N2$ displacement for each set is as follows: \begin{enumerate} \item 1-Bromopentane > 2-Bromopentane > 2-Bromo-2-methylbutane \item 1-Bromo-3-methylbutane > 2-Bromo-3-methylbutane > 2-Bromo-2-methylbutane \item 1-Bromobutane > 1-Bromo-3-methylbutane > 1-Bromo-2-methylbutane > 1-Bromo-2,2-dimethylpropane \end{enumerate}
Question17. Out of C6H5CH2Cl and C6H5CHClC6H5, which is more easily hydrolysed by aqueous KOH? : Haloalkanes and Haloarenes Chapter 6
Solution :
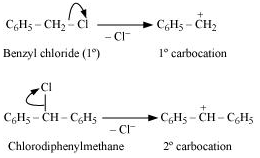
Hydrolysis by aqueous KOH proceeds through the formation of carbocation. If carbocation is stable, then the compound is easily hydrolyzed by aqueous KOH. Now, C6H5CH2Cl forms 1°-carbocation, while C6H5CHClC6H5
forms 2°-carbocation, which is more stable than 1°-carbocation. Hence, C6H5CHClC6H5 is hydrolyzed more easily than C6H5CH2Cl by aqueous KOH.
Question18. p-Dichlorobenzene has higher m.p. and lower solubility than those of o- and m-isomers. Discuss. : Haloalkanes and Haloarenes Chapter 6
Solution :

p-Dichlorobenzene is more symmetrical than o-and m-isomers. For this reason, it fits more closely than o-and m-isomers in the crystal lattice. Therefore, more energy is required to break the crystal lattice of p-dichlorobenzene. As a result, p-dichlorobenzene has a higher melting point and lower solubility than o-and m-isomers.
Question19. How the following conversions can be carried out?
(i) Propene to propan-1-ol
(ii) Ethanol to but-1-yne
(iii) 1-Bromopropane to 2-bromopropane
(iv) Toluene to benzyl alcohol
(v) Benzene to 4-bromonitrobenzene
(vi) Benzyl alcohol to 2-phenylethanoic acid
(vii) Ethanol to propanenitrile
(viii) Aniline to chlorobenzene
(ix) 2-Chlorobutane to 3, 4-dimethylhexane
(x) 2-Methyl-1-propene to 2-chloro-2-methylpropane
(xi) Ethyl chloride to propanoic acid
(xii) But-1-ene to n-butyliodide
(xiii) 2-Chloropropane to 1-propanol
(xiv) Isopropyl alcohol to iodoform
(xv) Chlorobenzene to p-nitrophenol
(xvi) 2-Bromopropane to 1-bromopropane
(xvii) Chloroethane to butane
(xviii) Benzene to diphenyl
(xix) tert-Butyl bromide to isobutyl bromide
(xx) Aniline to phenylisocyanide
Solution :
(i)

(ii)

(iii)

(iv)

(v)
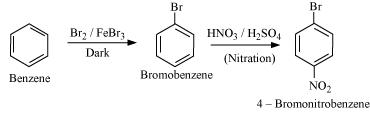
(vi)

(vii)
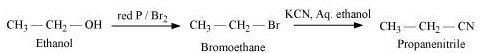
(viii)

(ix)

(x)
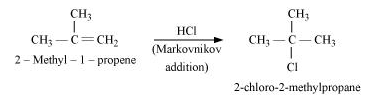
(xi)

(xii)

(xiii)

(xiv)

(xv)
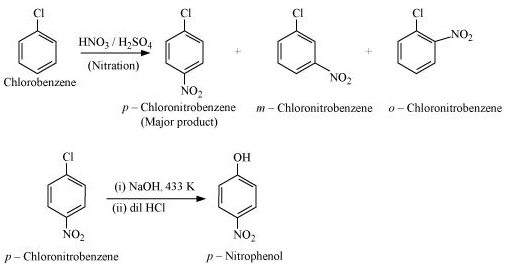
(xvi)

(xvii)

(xviii)

(xix)

(xx)

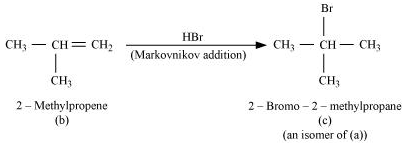
Question21. Primary alkyl halide C4H9Br (a) reacted with alcoholic KOH to give compound (b).Compound (b) is reacted with HBr to give (c) which is an isomer of (a). When (a) is reacted with sodium metal it gives compound (d), C8H18 which is different from the compound formed when n-butyl bromide is reacted with sodium. Give the structural formula of (a) and write the equations for all the reactions.
Solution :
There are two primary alkyl halides having the formula, C4H9Br. They are n − bulyl bromide and isobutyl bromide.

Therefore, compound (a) is either n−butyl bromide or isobutyl bromide.
Now, compound (a) reacts with Na metal to give compound (b) of molecular formula, C8H18, which is different from the compound formed when n−butyl bromide reacts with Na metal. Hence, compound (a) must be isobutyl bromide.

Thus, compound (d) is 2, 5−dimethylhexane.
It is given that compound (a) reacts with alcoholic KOH to give compound (b). Hence, compound (b) is 2−methylpropene.

Also, compound (b) reacts with HBr to give compound (c) which is an isomer of (a). Hence, compound (c) is 2−bromo−2−methylpropane.
Question22. What happens when
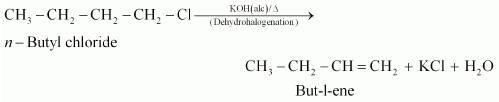
(i) n-butyl chloride is treated with alcoholic KOH,
(ii) bromobenzene is treated with Mg in the presence of dry ether,
(iii) chlorobenzene is subjected to hydrolysis,
(iv) ethyl chloride is treated with aqueous KOH,
(v) methyl bromide is treated with sodium in the presence of dry ether,
(vi) methyl chloride is treated with KCN.
Solution :
(i) When n−butyl chloride is treated with alcoholic KOH, the formation of but−l−ene takes place. This reaction is a dehydrohalogenation reaction.
(ii) When bromobenzene is treated with Mg in the presence of dry ether, phenylmagnesium bromide is formed.

(iii) Chlorobenzene does not undergo hydrolysis under normal conditions. However, it undergoes hydrolysis when heated in an aqueous sodium hydroxide solution at a temperature of 623 K and a pressure of 300 atm to form phenol.

(iv) When ethyl chloride is treated with aqueous KOH, it undergoes hydrolysis to form ethanol.

(v) When methyl bromide is treated with sodium in the presence of dry ether, ethane is formed. This reaction is known as the Wurtz reaction.

(vi) When methyl chloride is treated with KCN, it undergoes a substitution reaction to give methyl cyanide.
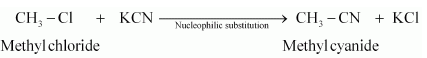
Question23. Write structures of the following compounds:
(i) 2-Chloro-3-methylpentane
(ii) 1-Chloro-4-ethylcyclohexane
(iii) 4-tert. Butyl-3-iodoheptane
(iv) 1,4-Dibromobut-2-ene
(v) 1-Bromo-4-sec. butyl-2-methylbenzene
Solution :
(i)
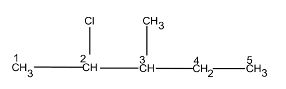
2-Chloro-3-methyl pentane
(ii)
1-Chloro-4-ethylcyclohexane
(iii)
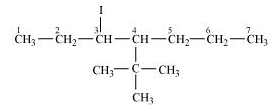
4- tert-Butyl-3-iodoheptane
(iv)
![]()
1, 4-Dibromobut-2-ene
(v)
1-Bromo-4-sec-butyl-2-methylbenzene
Question25. Write structures of different dihalogen derivatives of propane. : Haloalkanes and Haloarenes Chapter 6
Solution :
There are four different dihalogen derivatives of propane. The structures of these derivatives are shown below.
(i)
1, 1-Dibromopropane
(ii)
2, 2-Dibromopropane
(iii)
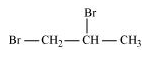
1, 2-Dibromopropane
(iv)
![]()
1, 3-Dibromopropane
Question27. Draw the structures of major monohalo products in each of the following reactions:

Solution :
Question28. Arrange each set of compounds in order of increasing boiling points. \begin{enumerate} \item Bromomethane, Bromoform, Chloromethane, Dibromomethane. \item 1-Chloropropane, Isopropyl chloride, 1-Chlorobutane. \end{enumerate} \section*
{Solution} \subsection*{(i)} For alkyl halides containing the same alkyl group, the boiling point increases with an increase in the atomic mass of the halogen atom. Therefore: \begin{itemize} \item The boiling point of bromomethane is higher than that of chloromethane due to the greater atomic mass of Br compared to Cl. \item The boiling point increases with the number of halogen atoms. Hence, dibromomethane has a higher boiling point than bromomethane and chloromethane but is lower than bromoform. \end{itemize} Thus, the order of increasing boiling points is: \[ \text{Chloromethane} < \text{Bromomethane} < \text{Dibromomethane} < \text{Bromoform} \] \subsection*{(ii)} For alkyl halides containing the same halide, the boiling point increases with an increase in the size of the alkyl group. Thus: \begin{itemize} \item 1-Chlorobutane has the highest boiling point due to the larger size of the alkyl group compared to 1-Chloropropane and Isopropyl chloride. \item Among 1-Chloropropane and Isopropyl chloride, the boiling point decreases with an increase in branching. Therefore, isopropyl chloride has a lower boiling point than 1-Chloropropane. \end{itemize} Thus, the order of increasing boiling points is: \[ \text{Isopropyl chloride} < \text{1-Chloropropane} < \text{1-Chlorobutane} \]
Question29. Which alkyl halide from the following pairs would you expect to react more rapidly by an SN2 mechanism? Explain your answer.

Solution :
(i)

2-bromobutane is a 2° alkylhalide whereas 1-bromobutane is a 1° alkyl halide. The approaching of nucleophile is more hindered in 2-bromobutane than in 1-bromobutane. Therefore, 1-bromobutane reacts more rapidly than 2-bromobutane by an SN2 mechanism.
(ii)
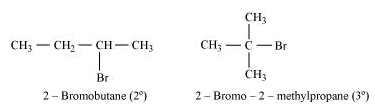
2-Bromobutane is 2° alkylhalide whereas 2-bromo-2-methylpropane is 3° alkyl halide. Therefore, greater numbers of substituents are present in 3° alkyl halide than in 2° alkyl halide to hinder the approaching nucleophile. Hence, 2-bromobutane reacts more rapidly than 2-bromo-2-methylpropane by an SN2 mechanism.
(iii)

Both the alkyl halides are primary. However, the substituent −CH3 is at a greater distance to the carbon atom linked to Br in 1-bromo-3-methylbutane than in 1-bromo-2-methylbutane. Therefore, the approaching nucleophile is less hindered in case of the former than in case of the latter. Hence, the former reacts faster than the latter by SN2 mechanism.
Question30. \begin{enumerate} \item In the following pairs of halogen compounds, which compound undergoes faster SN1 reaction? \item In the following pairs of halogen compounds, which compound undergoes faster SN1 reaction? \end{enumerate} \section*
{Solution} \subsection*{1} SN1 reactions proceed via the formation of a carbocation intermediate. The rate-determining step is the formation of this carbocation. Therefore, the stability of the carbocation affects the rate of the reaction: \begin{itemize} \item \textbf{2-chloro-2-methylpropane} forms a tertiary carbocation, which is highly stable. \item \textbf{3-chloropentane} forms a secondary carbocation, which is less stable than the tertiary carbocation. \end{itemize} Since the rate of the SN1 reaction is faster for compounds that form more stable carbocations, \textbf{2-chloro-2-methylpropane} undergoes the SN1 reaction faster than \textbf{3-chloropentane}. \subsection*{2} Similarly, for SN1 reactions: \begin{itemize} \item \textbf{2-chloroheptane} forms a secondary carbocation, which is more stable compared to a primary carbocation. \item \textbf{1-chlorohexane} forms a primary carbocation, which is less stable. \end{itemize} Thus, \textbf{2-chloroheptane} undergoes the SN1 reaction faster than \textbf{1-chlorohexane} due to the greater stability of the secondary carbocation.
Question 31. Identify A, B, C, D, E, R and R1 in the following:

Solution :

Since D of D2O gets attached to the carbon atom to which MgBr is attached, C is
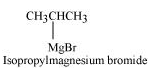
Therefore, the compound R − Br is

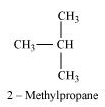
When an alkyl halide is treated with Na in the presence of ether, a hydrocarbon containing double the number of carbon atoms as present in the original halide is obtained as product. This is known as Wurtz reaction. Therefore, the halide, R1−X, is
Therefore, compound D is
And, compound E is

latest video
news via inbox
Nulla turp dis cursus. Integer liberos euismod pretium faucibua